|
Strain Name
|
C57BL/6-Cd3etm1(CD3E)Bcgen Cd3dtm1(CD3D)Bcgen
Cd3gtm1(CD3G)Bcgen Cd19tm4(CD19)Bcgen/Bcgen
|
Common Name
|
B-hCD3EDG/hCD19 mice
|
|
Background
|
C57BL/6
|
Catalog number
|
112902
|
Related Genes
|
CD3epsilon, IMD18, T3E, TCRE;
CD3-DELTAELTA, IMD19, T3D, CD3D;
CD3-GAMMAAMMA, IMD17, T3G, CD3G;
B4, CVID3;
|
NCBI Gene ID
|
916, 915, 917, 930
|
Description
-
CD3 consists of four protein chains (CD3E, CD3D, CD3G and CD3Z), which are important biological markers on the T cell membrane. CD3 can form a TCR/CD3 complex with the T cell receptor, participating in the regulation of T cell antigen recognition, signal transduction and T cell development. CD19 is a biomarker for normal and neoplastic B cells, as well as follicular dendritic cells. CD19 is critically involved in establishing intrinsic B cell signaling thresholds through modulating both B cell receptor-dependent and independent signaling.
-
B-hCD3EDG/hCD19 mice were obtained by mating B-hCD3EDG mice(110039) and B-hCD19 mice(112657). In B-hCD3EDG/hCD19 mice, chimeric human CD3EDG was expressed, while mouse Cd3edg were knocked out. The chimeric CD19 coding sequence containing the extracellular region of human CD19 gene is inserted into mouse Cd19 gene in B-hCD3EDG/hCD19 mice.
-
Human CD3E and CD19 can be detected respectively on T cells and B cells from spleen and blood of homozygous B-hCD3EDG/hCD19 mice, but not in wild-type (WT) mice. Humanization of CD3EDG and CD19 do not change the overall frequency or distribution of immune cell types in spleen, blood, thymus and lymph nodes.
-
This product is used for the pharmacological and safety evaluation of B-cell lymphoma and autoimmune diseases.
Protein expression analysis of CD19
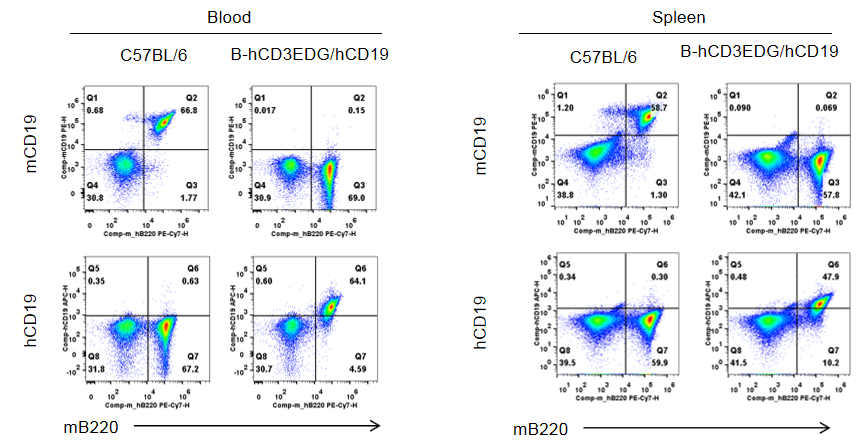
Strain specific CD19 expression analysis in wild-type C57BL/6 mice and homozygous humanized B-hCD3EDG/hCD19 mice by flow cytometry. Splenocytes and blood cells were collected from wild-type C57BL/6 mice and homozygous B-hCD3EDG/hCD19 mice (female, 7-week-old, n=1). Protein expression was analyzed with anti-mouse CD19 antibody (Biolegend, 115507) and anti-human CD19 antibody (Biolegend, 392503) by flow cytometry. Mouse CD19 was only detectable in wild-type C57BL/6 mice. Human CD19 was exclusively detectable in homozygous B-hCD3EDG/hCD19 mice, but not in wild-type C57BL/6 mice.
Protein expression analysis of CD3E
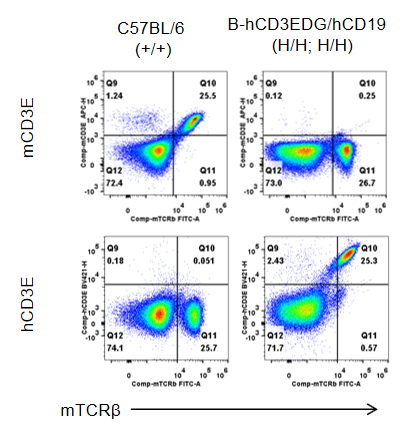
Strain specific CD3E expression analysis in wild-type C57BL/6 mice and homozygous humanized B-hCD3EDG/hCD19 mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice and homozygous B-hCD3EDG/hCD19 mice (female, 7-week-old, n=1). Protein expression was analyzed with anti-mouse CD3E antibody (Biolegend, 100312) and anti-human CD3E antibody (BD Horizon™, 562426) by flow cytometry. Mouse CD3E was only detectable in wild-type C57BL/6 mice. Human CD3E was exclusively detectable in homozygous B-hCD3EDG/hCD19 mice, but not in wild-type C57BL/6 mice.
Frequency of leukocyte subpopulations in spleen
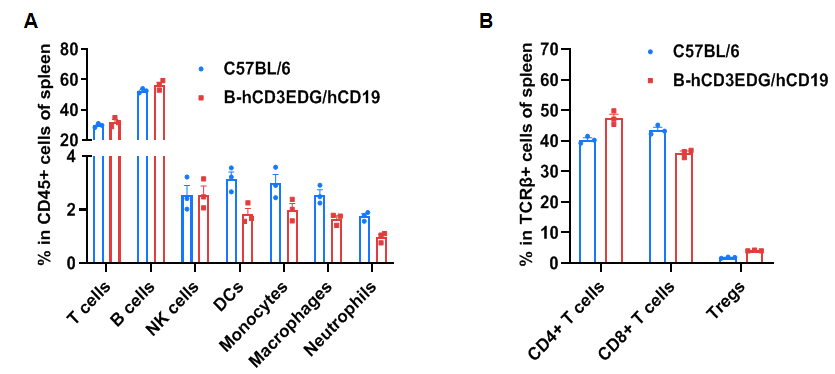
Frequency of leukocyte subpopulations in spleen by flow cytometry. Splenocytes were isolated from wild-type C57BL/6 mice and homozygous B-hCD3EDG/hCD19 mice (female, 8-week-old, n=3). A. Flow cytometry analysis of the splenocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percentages of T cells, B cells, NK cells, dendritic cells, monocytes, macrophages, neutrophils, CD4+ T cells, CD8+ T cells and Tregs in B-hCD3EDG/hCD19 mice were similar to those in C57BL/6 mice, demonstrating that humanization of CD3EDG and CD19 do not change the frequency or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
Frequency of leukocyte subpopulations in blood
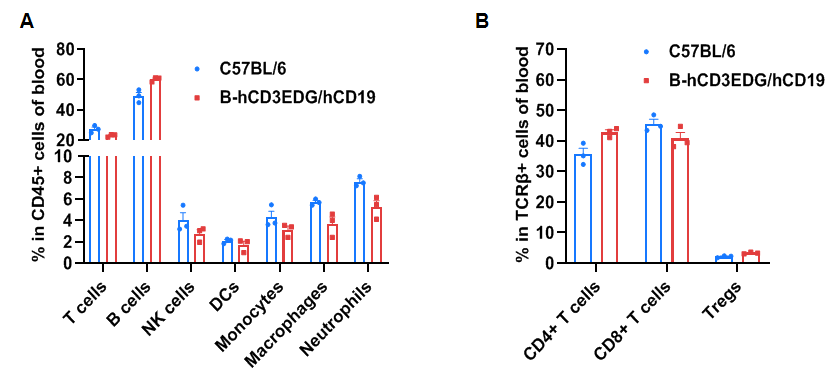
Frequency of leukocyte subpopulations in spleen by flow cytometry. Blood cells were isolated from wild-type C57BL/6 mice and homozygous B-hCD3EDG/hCD19 mice (female, 8-week-old, n=3). A. Flow cytometry analysis of the splenocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percentages of T cells, B cells, NK cells, dendritic cells, monocytes, macrophages, neutrophils, CD4+ T cells, CD8+ T cells and Tregs in B-hCD3EDG/hCD19 mice were similar to those in C57BL/6 mice, demonstrating that humanization of CD3EDG and CD19 do not change the frequency or distribution of these cell types in blood. Values are expressed as mean ± SEM.
Frequency of leukocyte subpopulations in lymph node
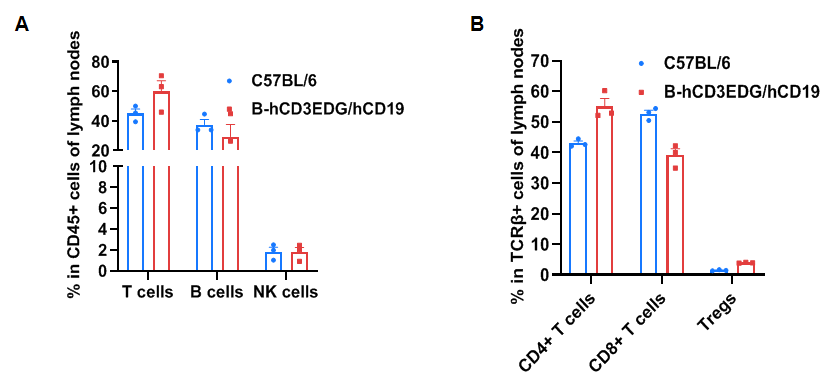
Frequency of leukocyte subpopulations in spleen by flow cytometry. Lymph nodes were isolated from wild-type C57BL/6 mice and homozygous B-hCD3EDG/hCD19 mice (female, 8-week-old, n=3). A. Flow cytometry analysis of the splenocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percentages of T cells, B cells, NK cells, CD4+ T cells, CD8+ T cells and Tregs in B-hCD3EDG/hCD19 mice were similar to those in C57BL/6 mice, demonstrating that humanization of CD3EDG and CD19 do not change the frequency or distribution of these cell types in lymph nodes. Values are expressed as mean ± SEM.
Frequency of leukocyte subpopulations in thymus
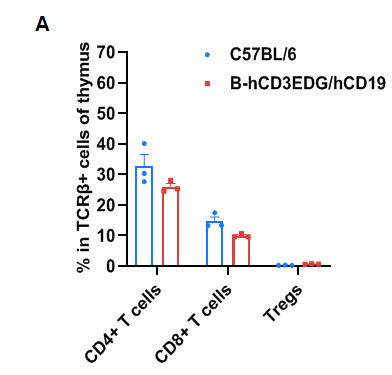
Frequency of leukocyte subpopulations in spleen by flow cytometry. Thymocytes were isolated from wild-type C57BL/6 mice and homozygous B-hCD3EDG/hCD19 mice (female, 8-week-old, n=3). A. Frequency of T cell subpopulations. Percentages of CD4+ T cells, CD8+ T cells and Tregs in B-hCD3EDG/hCD19 mice were similar to those in C57BL/6 mice, demonstrating that humanization of CD3EDG and CD19 do not change the frequency or distribution of these cell types in thymus. Values are expressed as mean ± SEM.
Hematology analysis

Complete blood count (CBC) of B-hCD3EDG/hCD19 mice. Values are expressed as mean ± SD.
Biochemistry analysis

Biochemical test of B-hCD3EDG/hCD19 mice. Values are expressed as mean ± SD.




















 京公网安备:
京公网安备: