|
Strain Name
|
C57BL/6-Pdcd1tm1(PDCD1)Bcgen Cd274tm1(CD274)Bcgen Tnfrsf18tm1(TNFRSF18) Bcgen/Bcgen
|
Common Name
|
B-hPD-1/hPD-L1/hGITR mice
|
|
Background
|
C57BL/6
|
Catalog number
|
131108
|
Related Genes
|
PDCD1 also known as PD1; PD-1; CD279; SLEB2; hPD-1; hPD-l; hSLE1.
CD274 also known as B7-H; B7H1; PDL1; PD-L1; hPD-L1; PDCD1L1; PDCD1LG1.
TNFRSF18 also known as AITR; GITR; CD357; GITR-D; ENERGEN
|
NCBI Gene ID
|
18566,60533,21936
|
Protein expression analysis

Strain specific PD-1 expression analysis in homozygous B-hPD-1/hPD-L1/hGITR mice by flow cytometry. Splenocytes were collected from wild-type mice (+/+) and homozygous B-hPD-1/hPD-L1/hGITR mice (H/H) stimulated with or without anti-CD3ε in vivo, and analyzed by flow cytometry with species-specific anti-PD-1 antibody. Mouse PD-1 was detectable in wild-type mice but not in homozygous B-hPD-1/hPD-L1/hGITR mice. Human PD-1 was exclusively detectable in homozygous B-hPD-1/hPD-L1/hGITR mice but not in wild-type mice.

Strain specific PD-L1 expression analysis in homozygous B-hPD-1/hPD-L1/hGITR mice by flow cytometry. Splenocytes were collected from wild-type mice (+/+) and homozygous B-hPD-1/hPD-L1/hGITR mice (H/H) stimulated with or without anti-CD3ε in vivo, and analyzed by flow cytometry with species-specific anti-PD-L1 antibody. Mouse PD-L1 was detectable in wild-type mice but not in homozygous B-hPD-1/hPD-L1/hGITR mice. Human PD-L1 was exclusively detectable in homozygous B-hPD-1/hPD-L1/hGITR mice but not in wild-type mice.
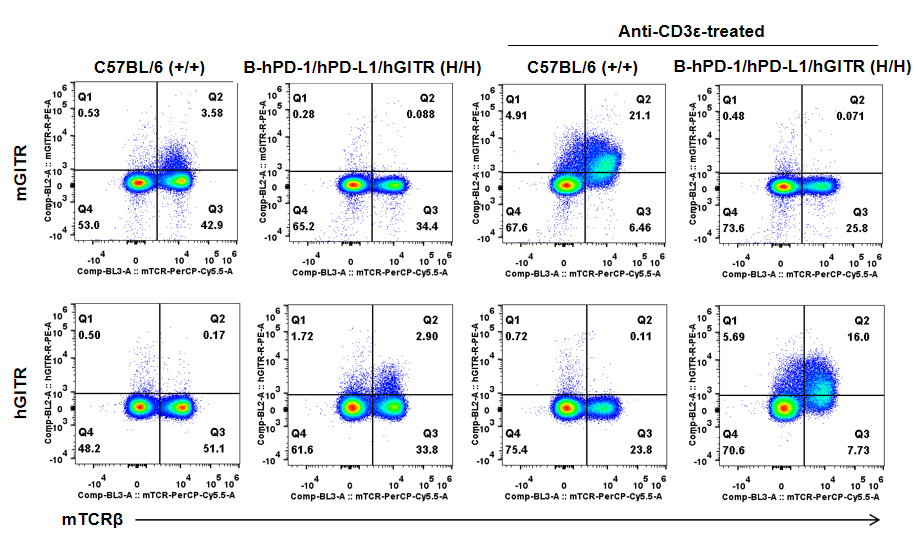
Strain specific GITR expression analysis in homozygous B-hPD-1/hPD-L1/hGITR mice by flow cytometry. Splenocytes were collected from wild-type mice (+/+) and homozygous B-hPD-1/hPD-L1/hGITR mice (H/H) stimulated with or without anti-CD3ε in vivo, and analyzed by flow cytometry with species-specific anti-GITR antibody. Mouse GITR was detectable in wild-type mice but not in homozygous B-hPD-1/hPD-L1/hGITR mice. Human GITR was exclusively detectable in homozygous B-hPD-1/hPD-L1/hGITR mice but not in wild-type mice.
Analysis of leukocytes subpopulation in B-hPD-1/hPD-L1/hGITR mice
Analysis of spleen leukocyte subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hPD-1/hPD-L1/hGITR mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes were performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hPD-1/hPD-L1/hGITR mice were similar to those in the C57BL/6 mice, demonstrating that PD-1/PD-L1/GITR humanized does not change the overall development, differentiation or distribution of these cell types in spleen. The same results were obtained in lymph nodes and blood (data not shown). Values are expressed as mean ± SEM.
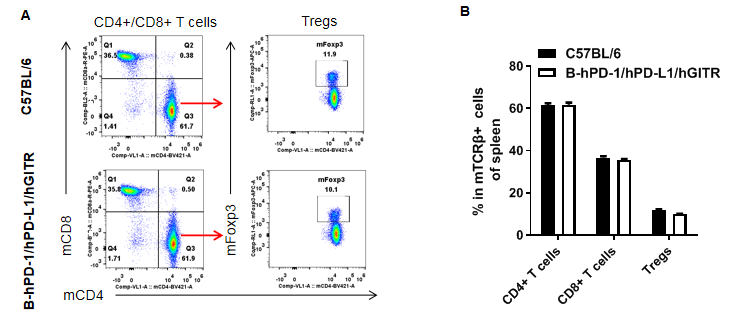
Analysis of spleen T cell subpopulations in B-hPD-1/hPD-L1/hGITR mice
Analysis of spleen T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hPD-1/hPD-L1/hGITR mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes ere performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD8+ T cells, CD4+ T cells and Tregs in homozygous B-hPD-1/hPD-L1/hGITR mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hPD-1/hPD-L1/hGITR in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in spleen. The same results were obtained in lymph nodes and blood (data not shown). Values are expressed as mean ± SEM.
Analysis of leukocytes subpopulation in B-hPD-1/hPD-L1/hGITR mice
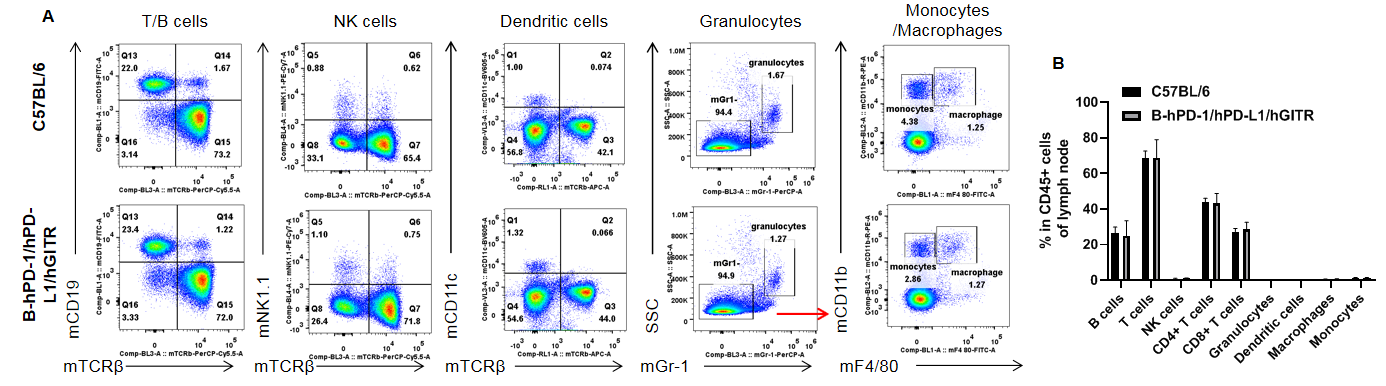
Analysis of lymph node leukocyte subpopulations by FACS. Lymph node was isolated from female C57BL/6 and B-hPD-1/hPD-L1/hGITR mice (n=3, 6-week-old). Flow cytometry analysis of the lymph node cells were performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hPD-1/hPD-L1/hGITR mice were similar to those in the C57BL/6 mice, demonstrating that PD-1/PD-L1/GITR humanized does not change the overall development, differentiation or distribution of these cell types in lymph node. Values are expressed as mean ± SEM.
Analysis of lymph node T cell subpopulations in B-hPD-1/hPD-L1/hGITR mice
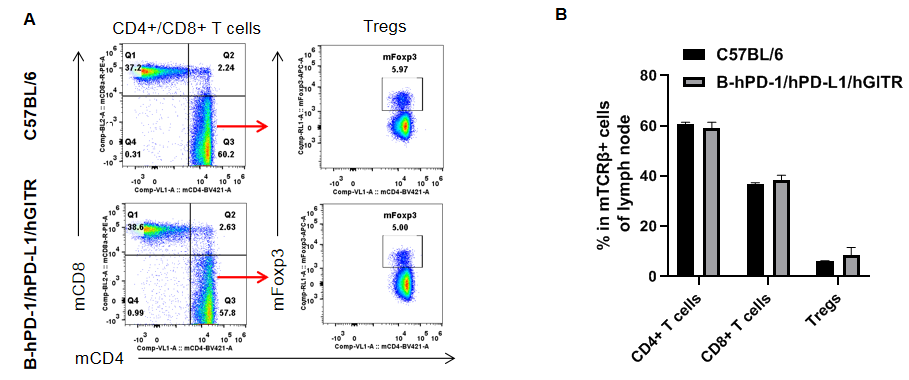
Analysis of lymph node T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hPD-1/hPD-L1/hGITR mice (n=3, 6-week-old). Flow cytometry analysis of the lymph node cells were performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD8+ T cells, CD4+ T cells, and Tregs in homozygous B-hPD-1/hPD-L1/hGITR mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hPD-1/hPD-L1/hGITR in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in lymph node. Values are expressed as mean ± SEM.
Analysis of leukocytes subpopulation in B-hPD-1/hPD-L1/hGITR mice
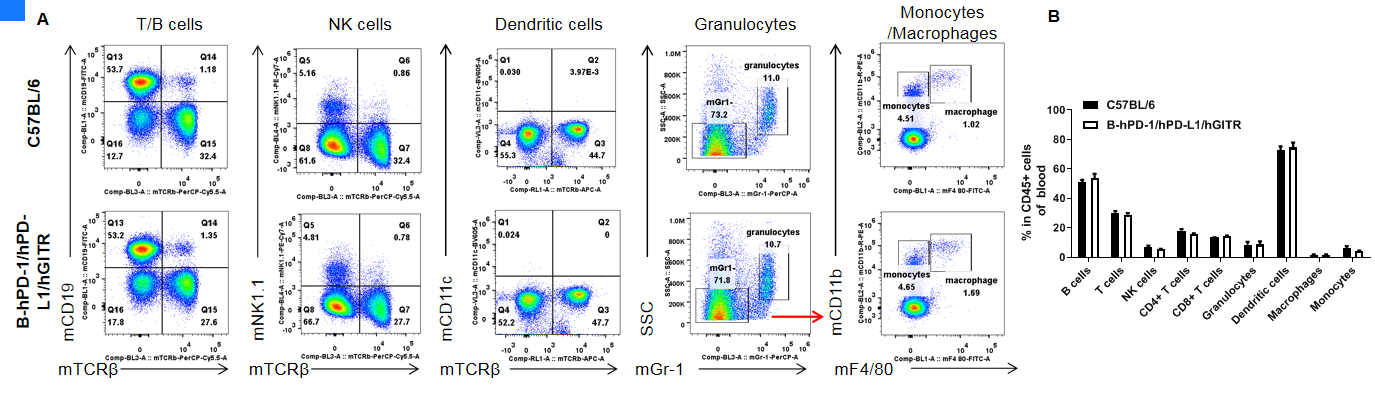
Analysis of blood leukocyte subpopulations by FACS. Blood cells were isolated from female C57BL/6 and B-hPD-1/hPD-L1/hGITR mice (n=3, 6-week-old). Flow cytometry analysis of the blood cells were performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hPD-1/hPD-L1/hGITR mice were similar to those in the C57BL/6 mice, demonstrating that PD-1/PD-L1/GITR humanized does not change the overall development, differentiation or distribution of these cell types in blood. Values are expressed as mean ± SEM.
Analysis of blood T cell subpopulations in B-hPD-1/hPD-L1/hGITR mice
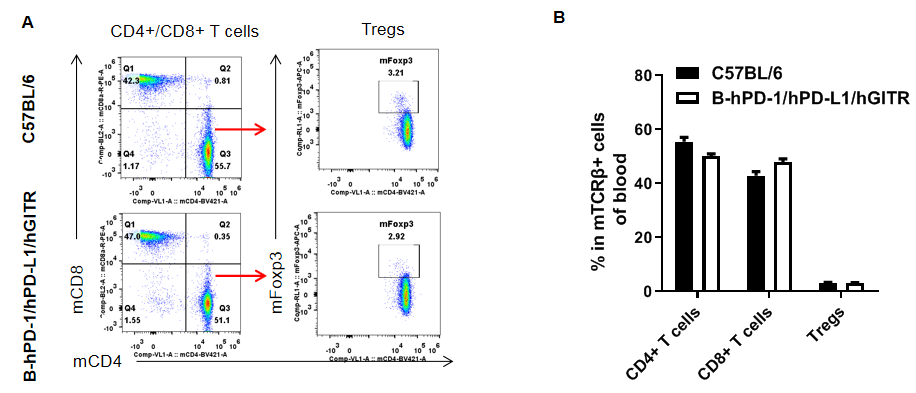
Analysis of blood T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hPD-1/hPD-L1/hGITR mice (n=3, 6-week-old). Flow cytometry analysis of the blood cells were performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD8+ T cells, CD4+ T cells, and Tregs in homozygous B-hPD-1/hPD-L1/hGITR mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hPD-1/hPD-L1/hGITR in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in blood. Values are expressed as mean ± SEM.
Combination therapy of anti-human PD-1 antibody and anti-human GITR antibody
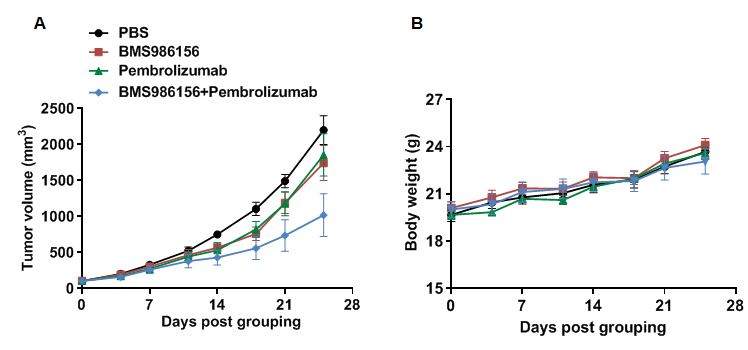
Antitumor activity of anti-human PD-1 antibody combined with anti-human GITR antibody in B-hPD-1/hPD-L1/hGITR mice. (A) Anti-human PD-1 antibody combined with anti-human GITR antibody inhibited MC38 tumor growth in B-hPD-1/hPD-L1/hGITR mice. All antibodies used in the experiment were prepared in-house. Murine colon cancer MC38 cells were subcutaneously implanted into homozygous B-hPD-1/hPD-L1/hGITR mice (female, 6-7 weeks old, n=5). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with human PD-1 and human GITR antibodies (in house) indicated in the panel. (B) Body weight changes during treatment. As shown in panel A, the antibodies were efficacious in controlling tumor growth in B-hPD-1/hPD-L1/hGITR mice, demonstrating that the B-hPD-1/hPD-L1/hGITR mice provide a powerful preclinical model for in vivo evaluation of PD-1 and GITR antibodies. Values are expressed as mean ± SEM.





















 京公网安备:
京公网安备: