| Strain Name |
C57BL/6-Tfrctm1(TFRC)Bcgen/Bcgen
|
Common Name | B-hTFR1 mice |
| Background | C57BL/6 | Catalog number |
110861 |
|
Related Genes |
T9; TR; TFR; p90; CD71; TFR1; TRFR; IMD46 |
||
|
NCBI Gene ID |
22042 | ||
mRNA expression analysis

Strain specific analysis of TFR1 gene expression in wild type (WT) mice and B-hTFR1 mice by RT-PCR. Mouse Tfrc mRNA was detectable only in splenocytes of WT mice (+/+). Human TFRC mRNA was detectable only in homozygous B-hTFR1 mice (H/H) but not in WT mice (+/+).
Protein expression analysis in erythroid cells

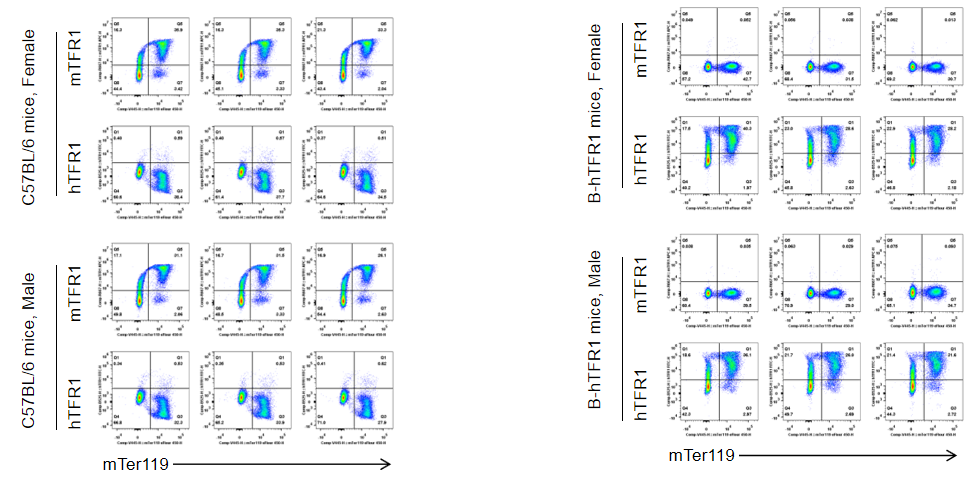
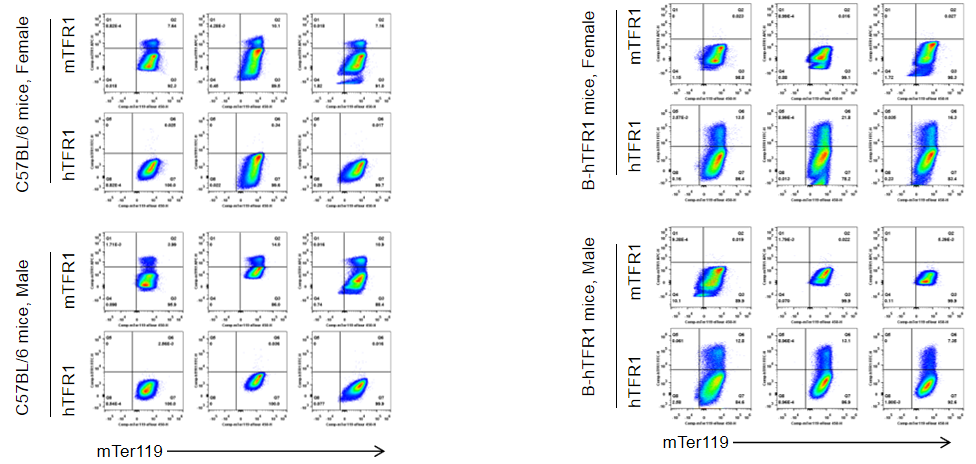
Strain specific TFR1 expression analysis in homozygous B-hTFR1 mice by flow cytometry. Bone marrow was collected from wild type (WT) mice (+/+) and homozygous B-hTFR1 mice (H/H), and analyzed by flow cytometry with species-specific anti-TFR1 antibody. Mouse TFR1 was detectable in WT mice (+/+). Human TFR1 was exclusively detectable in homozygous B-hTFR1 mice (H/H) but not in WT mice (+/+).
Protein expression analysis in brain
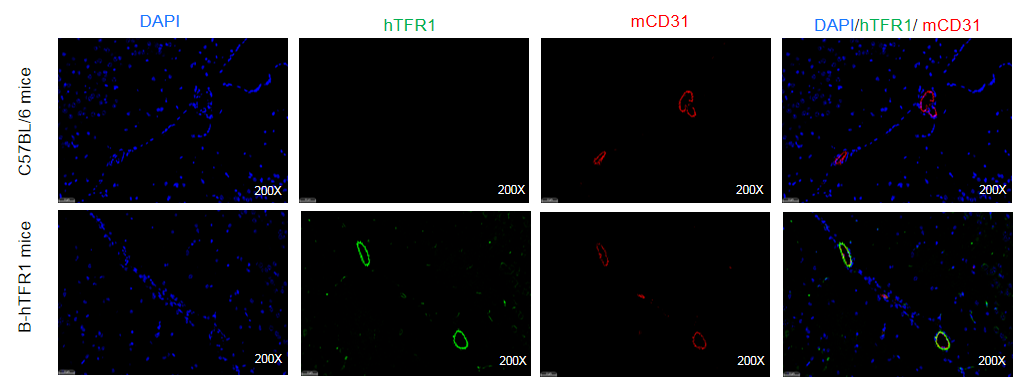
Strain specific TFR1 expression analysis in homozygous B-hTFR1 mice by Immunofluorescence staining. Brain was collected from wild-type C57BL/6 mice and homozygous B-hTFR1 mice (female,8-week-old) and processed into paraffin sections. The paraffin sections were stained using an species-specific anti-human TFR1 antibody (green). Brain tissues were co-stained with an anti-mouse CD31 antibody (red) to visualize microvascular endothelial cells. The results indicated that human TFR1 was detectable on brain microvascular endothelium of homozygous B-hTFR1 mice, but not in wild-type C57BL/6 mice.
Protein expression analysis in bone marrow
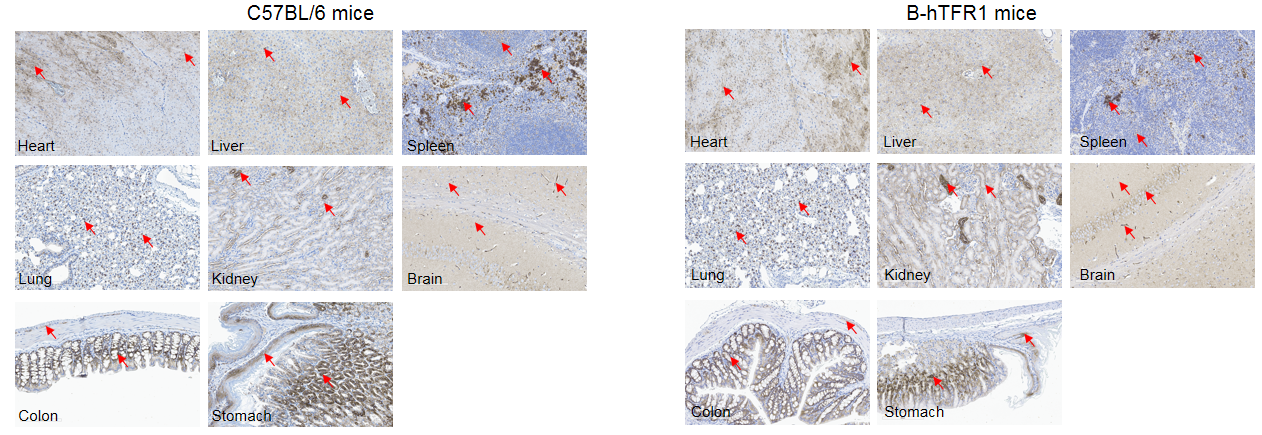
Immunohistochemical (IHC) analysis of TFR1 protein expression in wild-type C57BL/6 mice and homozygous humanized B-hTFR1 mice. Mouse tissues were collected from wild-type C57BL/6 mice and homozygous B-hTFR1 mice (female, 8-week-old, n=3). Protein expression was analyzed with anti-TFR1 antibody by IHC. Human TFR1 was detected in the heart, liver, spleen, lung, kidney, brain, colon and stomach of B-hTFR1 mice, which is similar to the expression pattern of mouse TFR1 in wild-type C57BL/6 mice. Red arrow: positive cells expressing TFR1.
Analysis of leukocytes cell subpopulations in B-hTFR1 mice

Humanization of TFR1 does not change the overall frequency or distribution of immune cell types in spleen, blood and lymph nodes. Values are expressed as mean ± SD.


Liver complement component 3 levels in wild-type C57BL/6 and homozygous humanized B-hTFR1 mice. Liver were collected from C57BL/6 and B-hTFR1 mice (n=3, 6 week old, female), then cDNA libraries were synthesized by reverse transcription, followed by qPCR with mouse complement component 3 primers. The mRNA expression of mouse complement component 3 in homozygous B-hTFR1 mice was similar to those in the wild-type C57BL/6 mice. Values are expressed as mean ± SEM. Significance was determined by unpaired t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
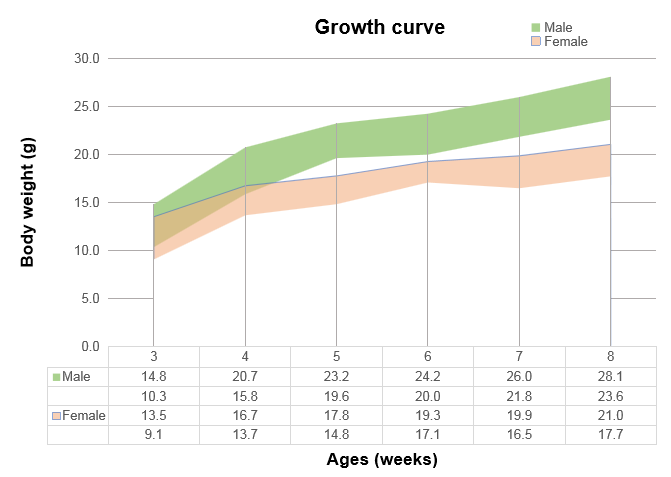

Humanization of TFR1 does not alter hematological parameters. Values are expressed as mean ± SD.
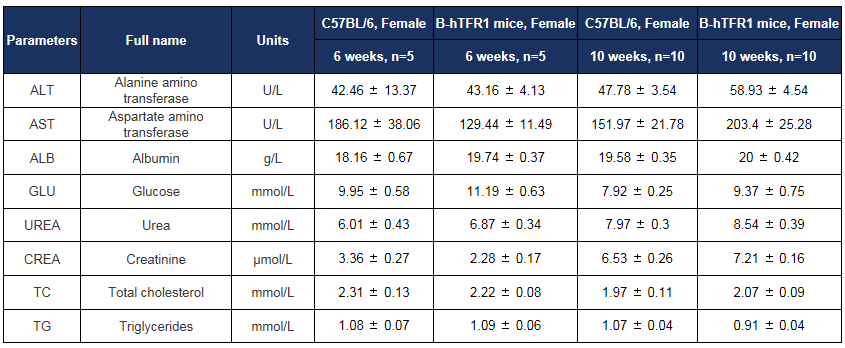
Biochemical test of B-hTFR1 mice. Values are expressed as mean ± SD.
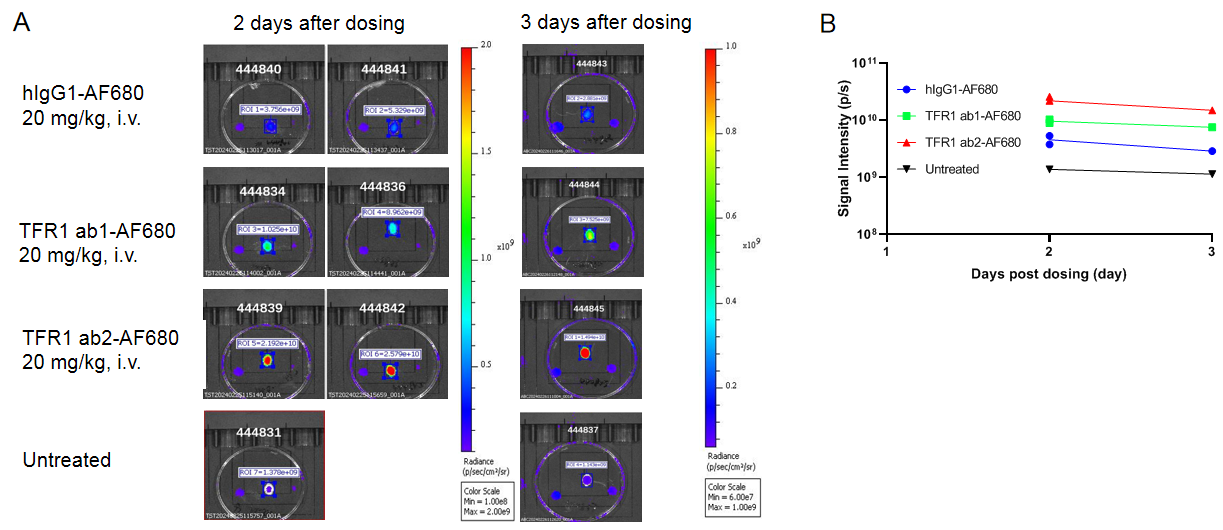
In vivo PK evaluation of anti-human TFR1 BsAbs

In vivo pharmacokinetic (PK) evaluation of anti-human TFR1 bispecific antibodies (BsAbs). B-hTFR1 mice were injected with control IgG (10 mpk) and anti-human TFR1 BsAbs (10.9 mpk) provided by a client via tail vein. Brain and serum were taken for in vivo PK evaluation. Brain concentrations(A), serum concentrations (B), and brain-to-serum ratio (C) of anti-human TFR1 BsAbs were quantified. As shown in panel, anti-human TFR1 BsAbs exhibited higher serum clearance and enhanced brain exposure after dose. The results confirmed that brain of B-hTFR1 mice enables uptake of an intravenously administered anti-human TFR1 BsAbs and B-hTFR1 mice provide a powerful preclinical model for in vivo evaluation of effective delivery of protein therapeutics to the central nervous system (CNS). Graphs represent mean ± SEM.
Note: This experiment was performed by the client using B-hTFR1 mice. All the other materials were provided by the client.












 京公网安备:
京公网安备: