• 321957
The mouse Met gene was replaced by human MET coding sequence in B-hMET MC38 cells. Human MET is highly expressed on the surface of B-hMET MC38 cells.
Gene targeting strategy for B-hMET MC38 cells. The exogenous promoter and human MET coding sequence was inserted to replace part of murine exon 3. The insertion disrupts the endogenous murine Met gene, resulting in a non-functional transcript.
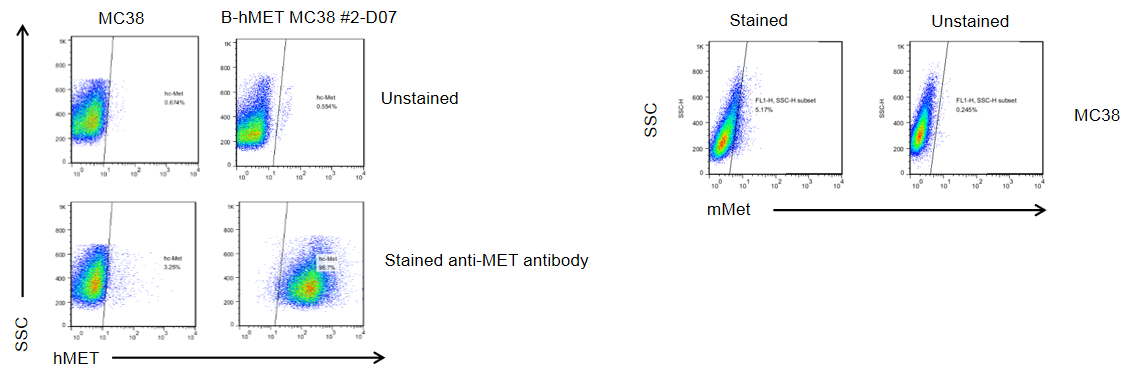
MET expression analysis in B-hMET MC38 cells by flow cytometry. Single cell suspensions from wild-type MC38 and B-hMET MC38 cultures were stained with species-specific anti-MET antibody. Mouse MET was not detected on the surface of MC38 cells. Human MET was detected on the surface of B-hMET MC38 cells but not wild-type MC38 cells. The 2-D07 clone of B-hMET MC38 cells was used for in vivo experiments.
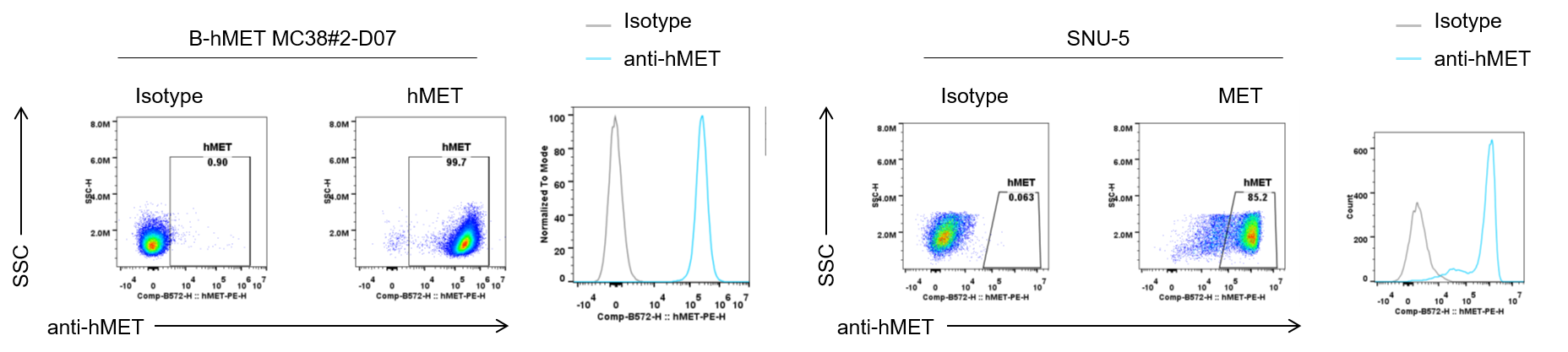
Binding of an anti-human MET antibody to B-hMET MC38 cells. B-hMET MC38#2-D07 cells and the positive control SNU-5 cell line were collected and analyzed by flow cytometry using anti-hMET antibody (telisotuzumab, in-house). The antibody bound to both B-hMET MC38 and SNU-5 cells.
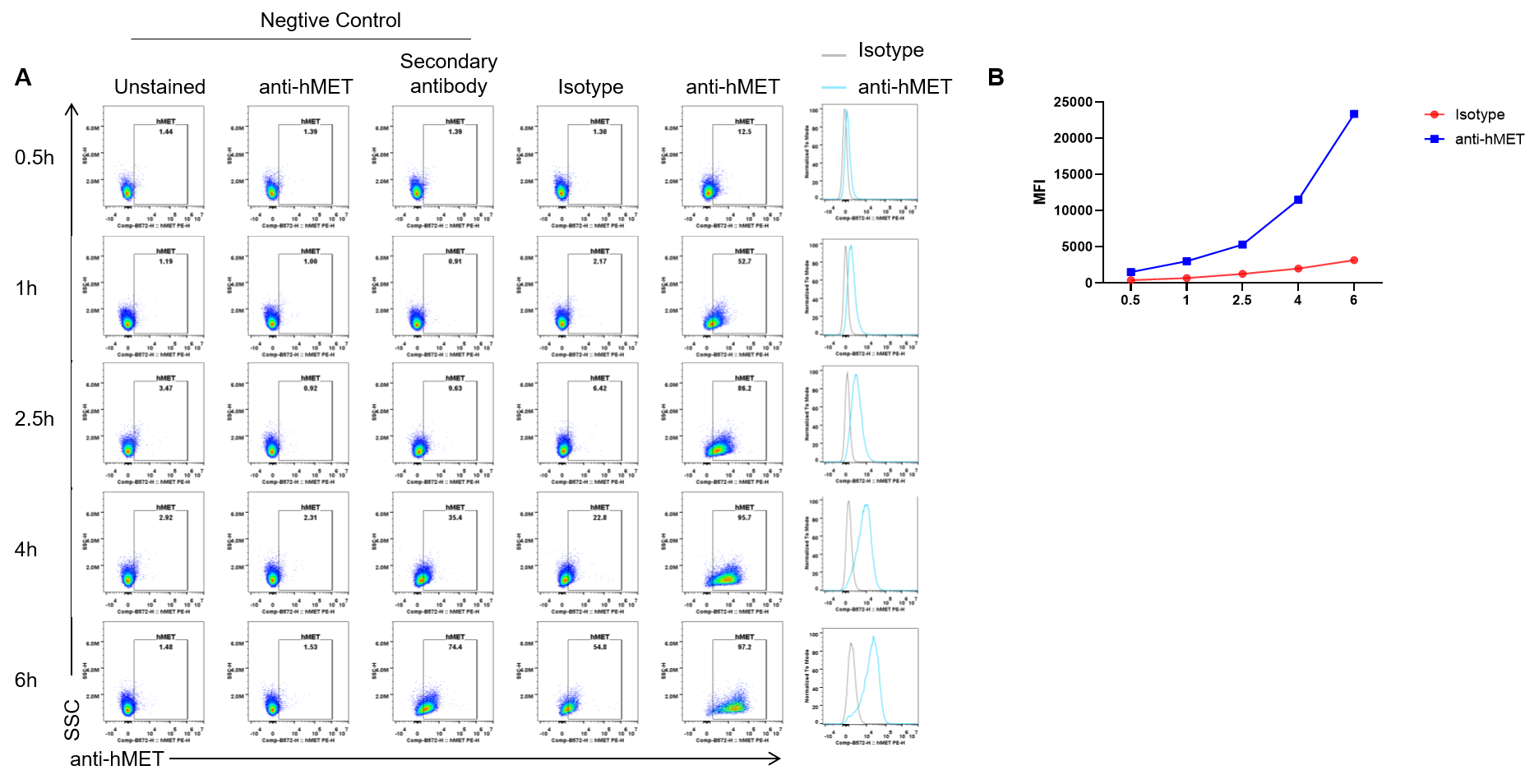
Endocytosis experiment results analysis in B-hMET MC38 cells by flow cytometry. B-hMET-MC38 #2-D07 cells were incubated for a set time with a pre-formed complex of anti-hMET antibody (telisotuzumab, in-house ) and a fluorophore-conjugated secondary antibody. Following incubation, cellular uptake of the antibody complex was measured by flow cytometry to assess internalization capability. The results demonstrate efficient uptake in these cells (A, B).
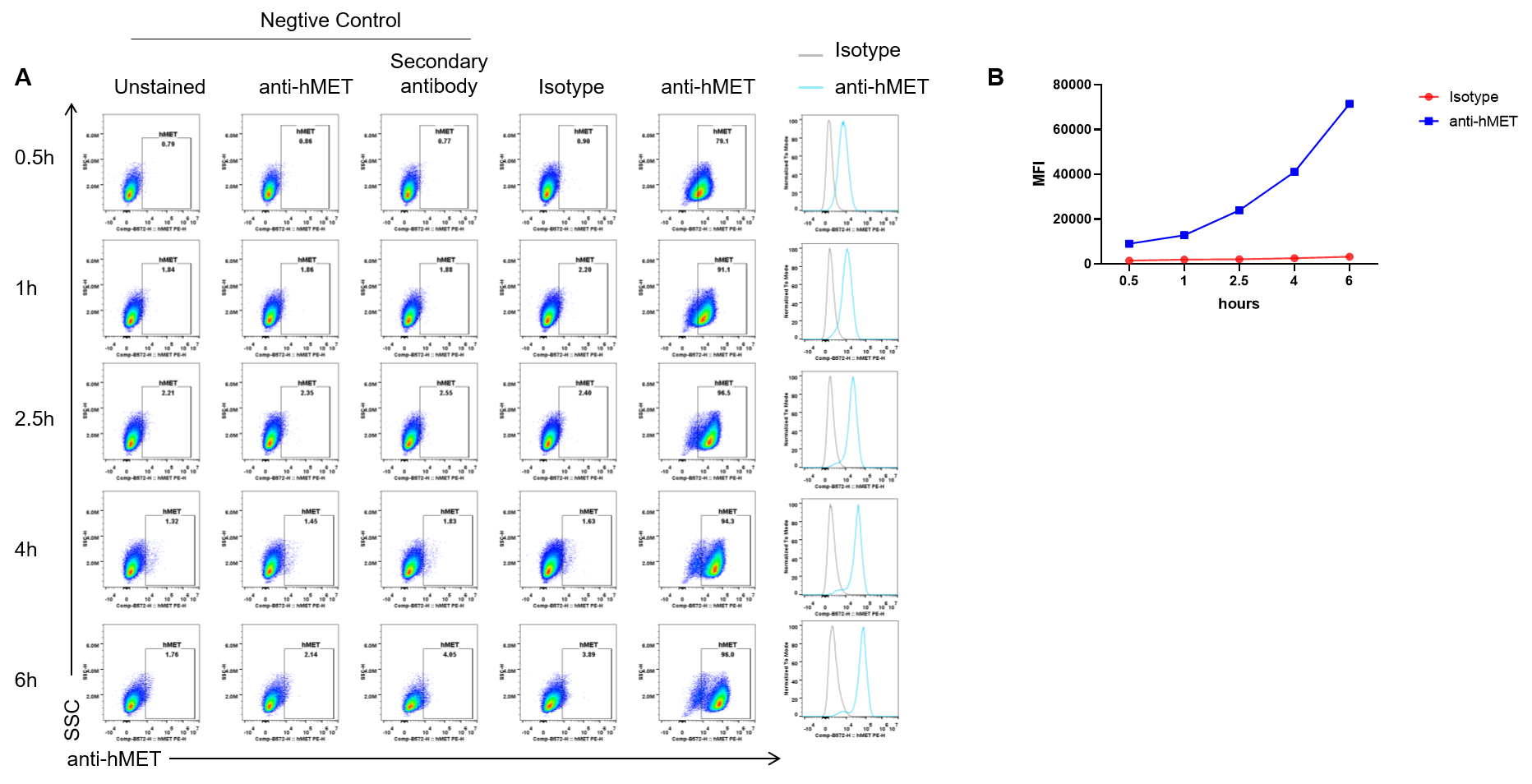
Endocytosis experiment results analysis in SNU-5 cells by flow cytometry. SNU-5 cells were incubated for a set time with a pre-formed complex of anti-hMET antibody (telisotuzumab, in-house ) and a fluorophore-conjugated secondary antibody. Following incubation, cellular uptake of the antibody complex was measured by flow cytometry to assess internalization capability. The results demonstrate efficient uptake in these cells (A, B).
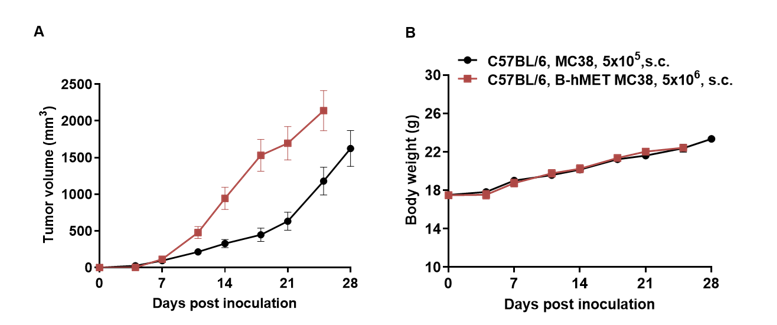
Subcutaneous homograft tumor growth of B-hMET MC38 cells. B-hMET MC38 cells (5x106) and wild-type MC38 cells (5x105) were subcutaneously implanted into C57BL/6 mice (female, 7-week-old). Tumor volume and body weight were measured twice a week. (A) Average tumor volume ± SEM. (B) Body weight (Mean± SEM). Volume was expressed in mm3 using the formula: V=0.5 X long diameter X short diameter2. As shown in panel A, B-hMET MC38 cells were able to establish tumors in vivo and can be used for efficacy studies.
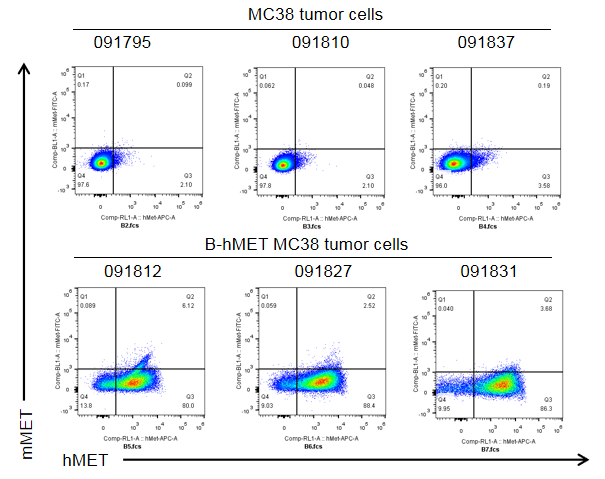
B-hMET MC38 cells were subcutaneously transplanted into C57BL/6 mice. At the end of the experiment, tumor cells were harvested and assessed for human MET expression by flow cytometry. As shown, human MET was highly expressed on the surface of tumor cells. Therefore, B-hMET MC38 cells can be used for in vivo efficacy studies of novel MET therapeutics.