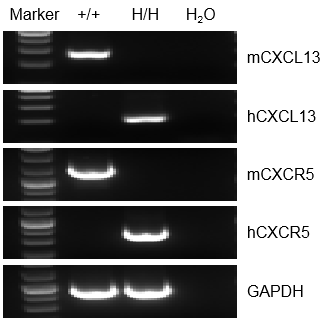
mRNA expression analysis
Strain specific analysis of CXCL13 and CXCR5 gene expression in WT and homozygous B-hCXCL13/hCXCR5 mice by RT-PCR. Mouse Cxcl13 and Cxcr5 mRNA were detectable in splenocytes of wild-type (+/+) mice. Human CXCL13 and CXCR5 mRNA were detectable only in homozygous B-hCXCL13/hCXCR5 mice , but not in wild-type (+/+) mice.
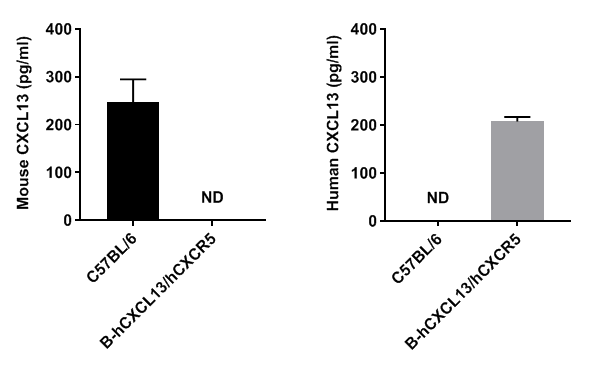
Protein expression analysis
Strain specific CXCL13 expression analysis in homozygous B-hCXCL13/hCXCR5 mice by ELISA. Serum were collected from WT and homozygous B-hCXCL13/hCXCR5 mice, and analyzed by ELISA with species-specific CXCL13 ELISA kit. Mouse CXCL13 was detectable in WT mice. Human CXCL13 was exclusively detectable in homozygous B-hCXCL13/hCXCR5 but not WT mice.
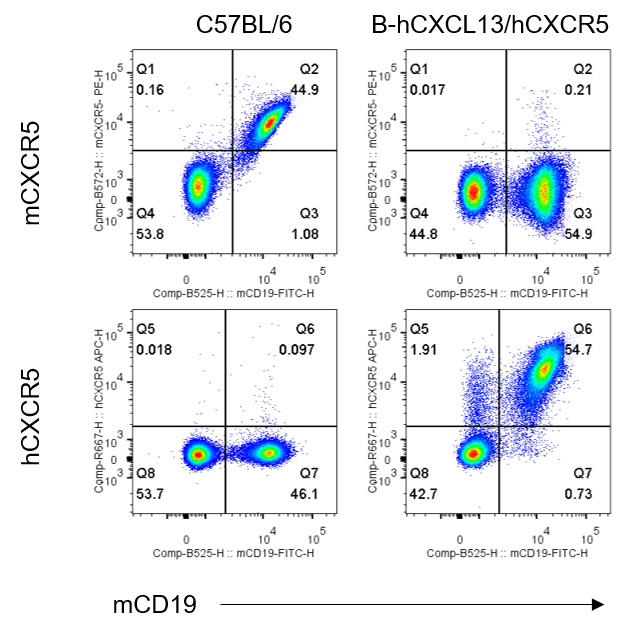
Strain specific CXCR5 expression analysis in homozygous B-hCXCL13/hCXCR5 mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice and homozygous B-hCXCL13/hCXCR5 mice, and analyzed by flow cytometry with anti-mouse CXCR5 antibody (Biolegend, 145503) and anti-human CXCR5 antibody (Biolegend, 356907) . Mouse CXCR5 was detectable in wild-type C57BL/6 mice. Human CXCR5 was exclusively detectable in homozygous B-hCXCL13/hCXCR5 mice but not in wild-type C57BL/6 mice.
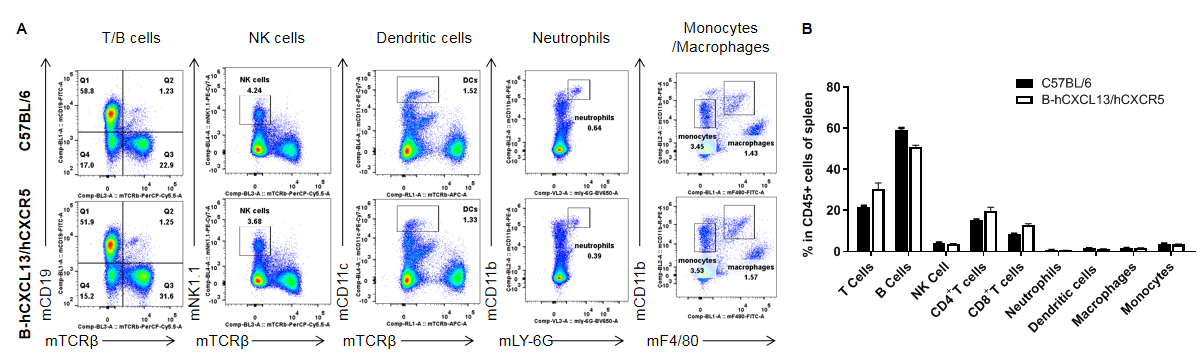
Analysis of leukocytes cell subpopulation in spleen
Analysis of spleen leukocyte subpopulations by FACS. Splenocytes were collected from WT and homozygous B-hCXCL13/hCXCR5 mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, neutrophils, monocytes and macrophages in homozygous B-hCXCL13/hCXCR5 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCXCL13/hCXCR5 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
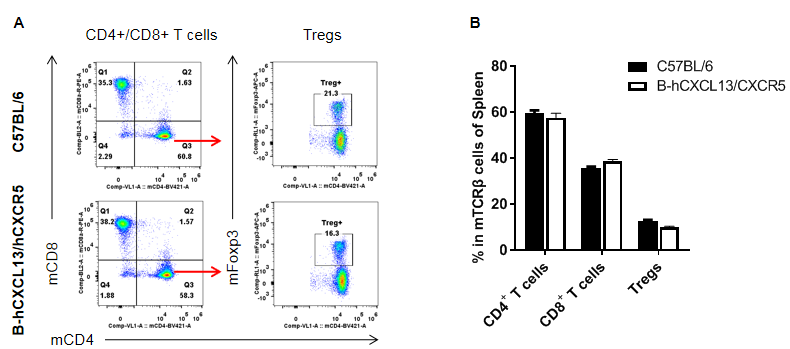
Analysis of T cell subpopulation in spleen
Analysis of spleen T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and homozygous B-hCXCL13/hCXCR5 mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD8+ T cells, CD4+ T cells, and Tregs in homozygous B-hCXCL13/hCXCR5 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCXCL13/hCXCR5 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in spleen. Values are expressed as mean ± SEM.
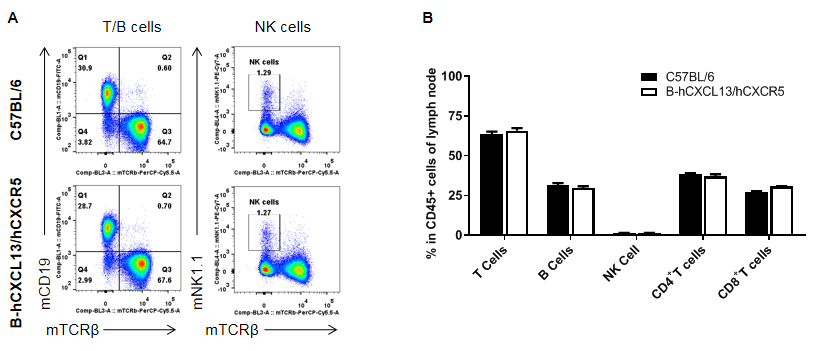
Analysis of leukocytes cell subpopulation in lymph node
Analysis of lymph node leukocyte subpopulations by FACS. Leukocytes were collected from WT and homozygous B-hCXCL13/hCXCR5 mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of T cells, B cells and NK cells in homozygous B-hCXCL13/hCXCR5 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCXCL13/hCXCR5 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in lymph node. Values are expressed as mean ± SEM.
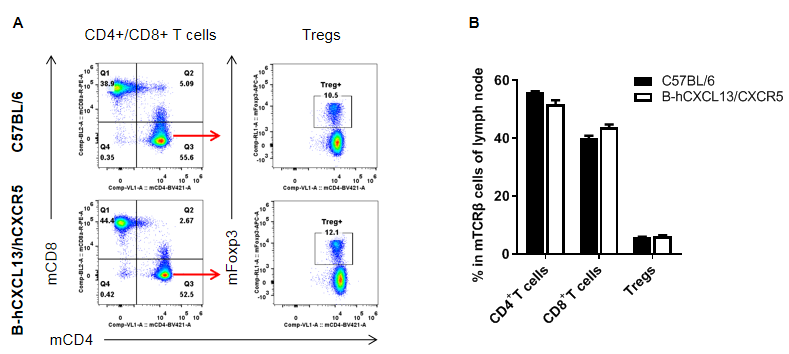
Analysis of T cell subpopulation in lymph node
Analysis of lymph node T cell subpopulations by FACS. Leukocytes were isolated from female C57BL/6 and homozygous B-hCXCL13/hCXCR5 mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD8+ T cells, CD4+ T cells, and Tregs in homozygous B-hCXCL13/hCXCR5 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCXCL13/hCXCR5 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in lymph node. Values are expressed as mean ± SEM.
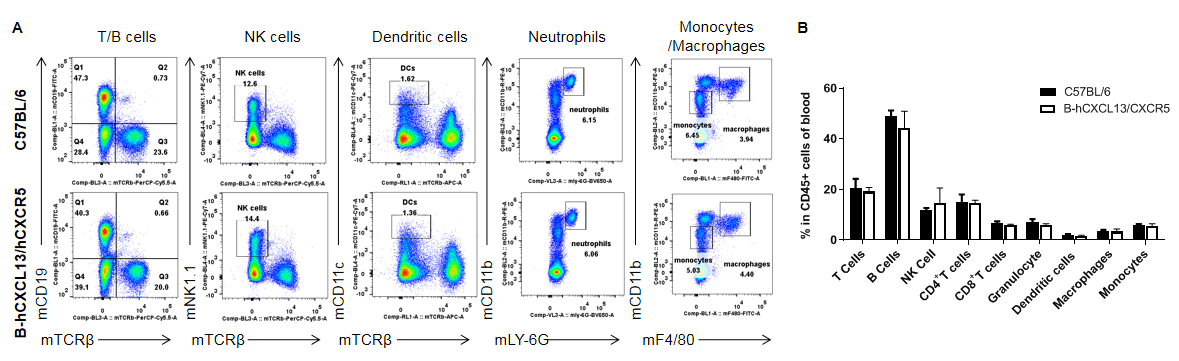
Analysis of leukocytes cell subpopulation in blood
Analysis of blood leukocyte subpopulations by FACS. Blood were collected from WT and homozygous B-hCXCL13/hCXCR5 mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, neutrophils, monocytes and macrophages in homozygous B-hCXCL13/hCXCR5 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCXCL13/hCXCR5 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in blood. Values are expressed as mean ± SEM.
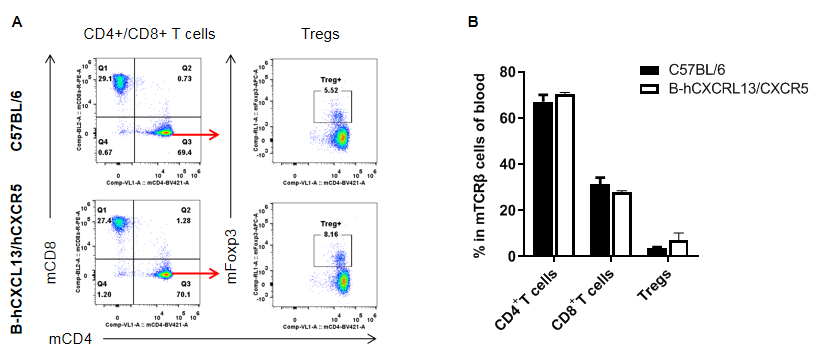
Analysis of T cell subpopulation in blood
Analysis of blood T cell subpopulations by FACS. Blood were isolated from female C57BL/6 and homozygous B-hCXCL13/hCXCR5 mice (n=3, 6-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD8+ T cells, CD4+ T cells, and Tregs in homozygous B-hCXCL13/hCXCR5 mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCXCL13/hCXCR5 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in blood. Values are expressed as mean ± SEM.
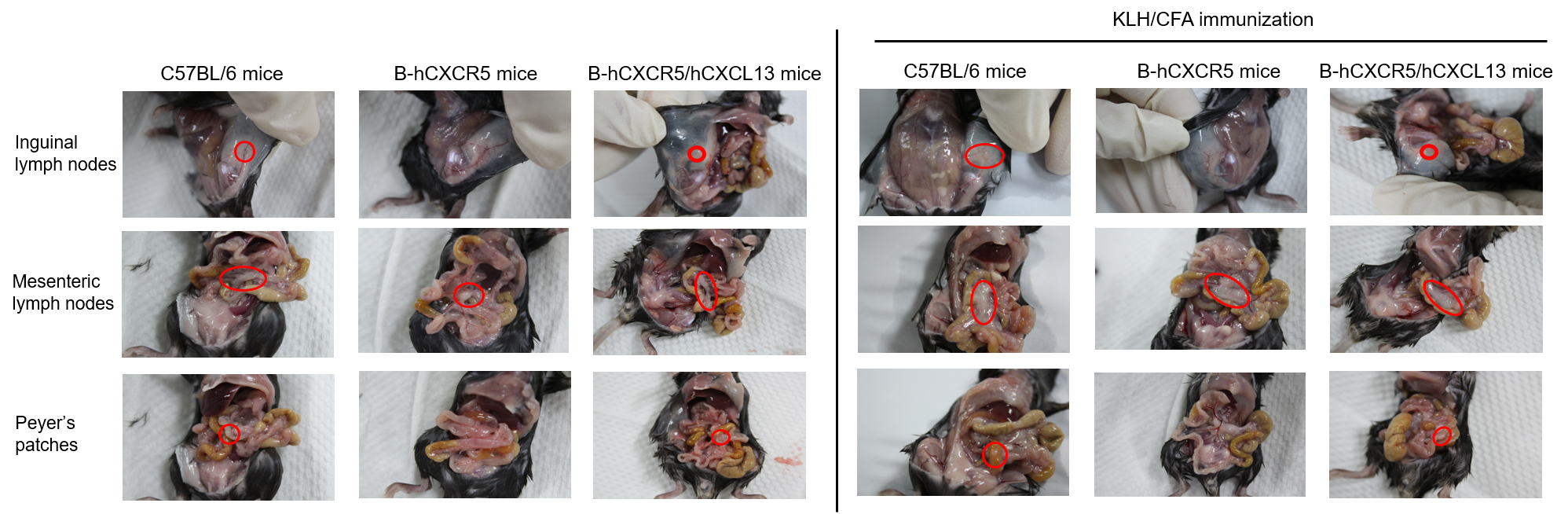
Observation of lymph nodes
C57BL/6 mice, B-hCXCR5 mice and B-hCXCR5/hCXCL13 mice (female, 8-week-old, n=3) were immunized intraperitoneally with 200 μg keyhole limpet hemocyanin (KLH) protein emulsified in complete Freund's adjuvant (CFA). After 14 days of immunization, the lymph nodes were observed and the spleen was analyzed by hematologic and epithelial analysis (H&E). As shown in the figure, inguinal lymph nodes, mesenteric lymph nodes, and Peyer's patches were clearly observed in C57BL/6 mice and B-hCXCR5/hCXCL13 mice. Inguinal lymph nodes and Peyer's patches were not observed in B-hCXCR5 mice, similar to the phenotype of CXCR5 knockout mice.
Note: CXCR5 homozygous null mutants lack inguinal lymph nodes, have a few abnormal or no Peyer's patches, morphologically altered primary lymphoid follicles and no functional germinal centers in their spleen.(https://www.informatics.jax.org/marker/MGI:103567)
Detection of the germinal center in spleen
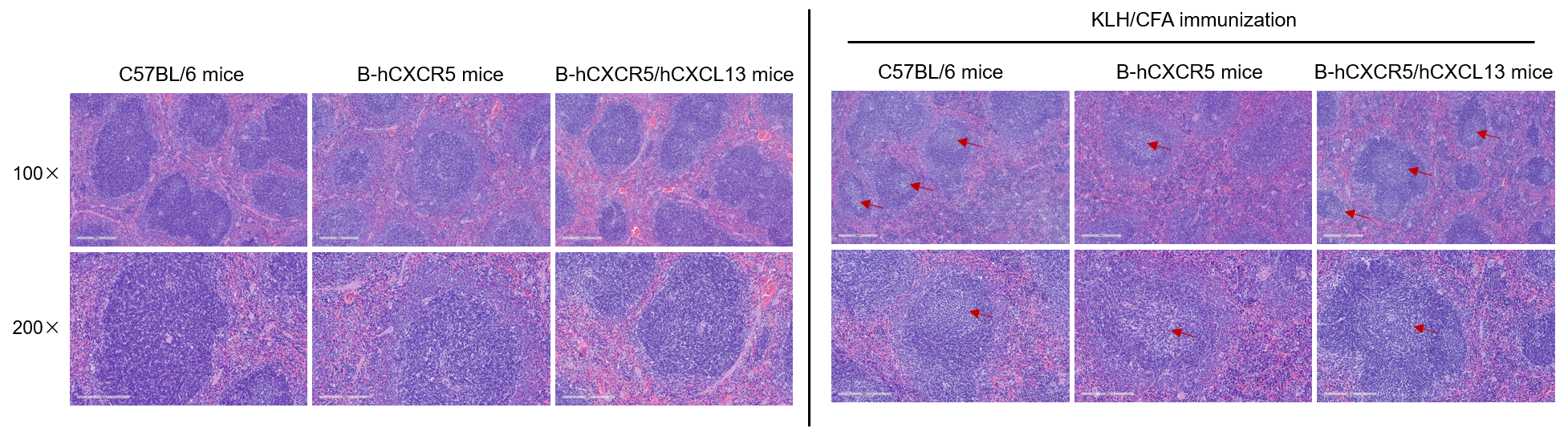
C57BL/6 mice, B-hCXCR5 mice and B-hCXCR5/hCXCL13 mice (female, 8-week-old, n=3) were immunized intraperitoneally with 200 μg keyhole limpet hemocyanin (KLH) protein emulsified in complete Freund's adjuvant (CFA). After 14 days of immunization, the lymph nodes were observed and the spleen was analyzed by hematologic and epithelial analysis (H&E). As shown in the figure, no obvious germinal centers were observed in the spleen of healthy mice that did not been immunized with KLH. Obvious germinal centers were observed in the spleen of KLH-immunized C57BL/6 mice, B-hCXCR5 mice, and B-hCXCR5/hCXCL13 mice, although the number of germinal centers was less in B-hCXCR5 mice. The red arrows indicate the germinal centers. This indicates that the humanized CXCR5 and CXCL13 pathways are normal and do not affect the formation of germinal centers in the mouse spleen.
Note: CXCR5 homozygous null mutants lack inguinal lymph nodes, have a few abnormal or no Peyer's patches, morphologically altered primary lymphoid follicles and no functional germinal centers in their spleen.(https://www.informatics.jax.org/marker/MGI:103567)
* When publishing results obtained using this animal model, please acknowledge the source as follows: The animal model [B-hCXCL13/hCXCR5 mice] (Cat# 111189) was purchased from Biocytogen.