
C57BL/6-Igf1rtm5(IGF1R)Bcgen/Bcgen • 111974
Background:
This receptor binds insulin-like growth factor with a high affinity. It has tyrosine kinase activity. The insulin-like growth factor I receptor plays a critical role in transformation events. Cleavage of the precursor generates alpha and beta subunits. It is highly overexpressed in most malignant tissues where it functions as an anti-apoptotic agent by enhancing cell survival.
Targeting strategy:
Validation:
Application:
For example, this product can be used for pharmacodynamics and safety evaluation of antibody drugs for cancers. Also, this product can be used to evaluate the pharmacodynamics and safety of treatments for tumors and neurodegenerative diseases, as well as to assess the potential of drugs to penetrate the blood-brain barrier.
Gene targeting strategy for B-hIGF1R mice plus. A chimeric CDS that encodes mouse Igf1r signal peptide and human extracellular domain, human IGF1R transmembrane and mouse cytoplasmic domain, followed by mouse Igf1r 3’UTR region, are inserted right after mouse Igf1r exon 2 to replace the exon 2 of mouse Igf1r gene. The chimeric IGF1R protein expression will be driven by endogenous mouse Igf1r promoter, while mouse Igf1r gene transcription and translation will be disrupted.
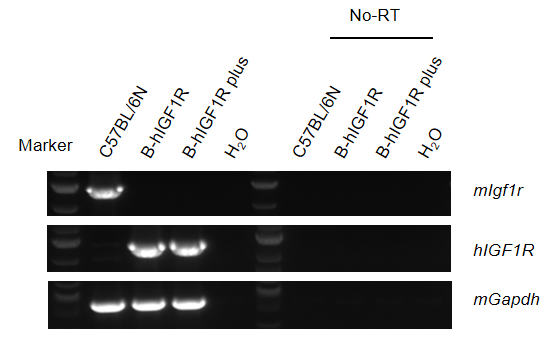
Strain specific analysis of IGF1R mRNA expression in wild-type C57BL/6N mice and B-hIGF1R mice by RT-PCR. Kidney RNA were isolated from wild-type C57BL/6N mice (+/+) (n=1, 14-week-old, male) and homozygous B-hIGF1R mice (H/H) (n=1, 8-week-old, male) and homozygous B-hIGF1R mice plus (H/H) (n=1, 14-week-old, male), then cDNA libraries were synthesized by reverse transcription, followed by PCR with IGF1R primers. Mouse Igf1r was only detectable in wild-type C57BL/6N mice. Human IGF1R was exclusively detectable in homozygous B-hIGF1R mice and homozygous B-hIGF1R mice plus but not in wild-type mice. No-RT: no reverse-transcripted.
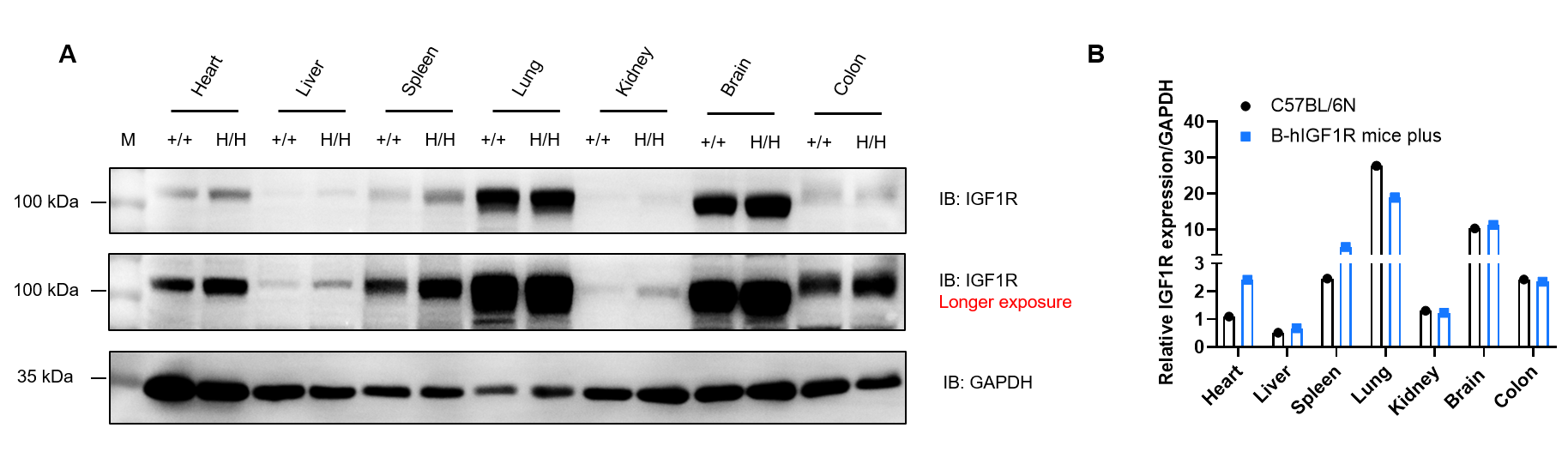
Western blot analysis of IGF1R protein expression in wild-type C57BL/6N mice and homozygous B-hIGF1R mice plus by WB. Various tissues were collected from wild-type C57BL/6N mice (+/+) and homozygous B-hIGF1R mice plus (H/H), and then analyzed by western blot with anti-IGF1R antibody (CST, 3027S). 40 μg total proteins were loaded for western blotting analysis. GAPDH were detected as internal control. (A) IGF1R was detectable in heart, liver, spleen, lung, kidney, brain and colon from both C57BL/6N and homozygous B-hIGF1R mice plus, as the antibody was cross-reactive between human and mouse. M, marker. (B) Relative quantification of IGF1R protein levels normalized to GAPDH.
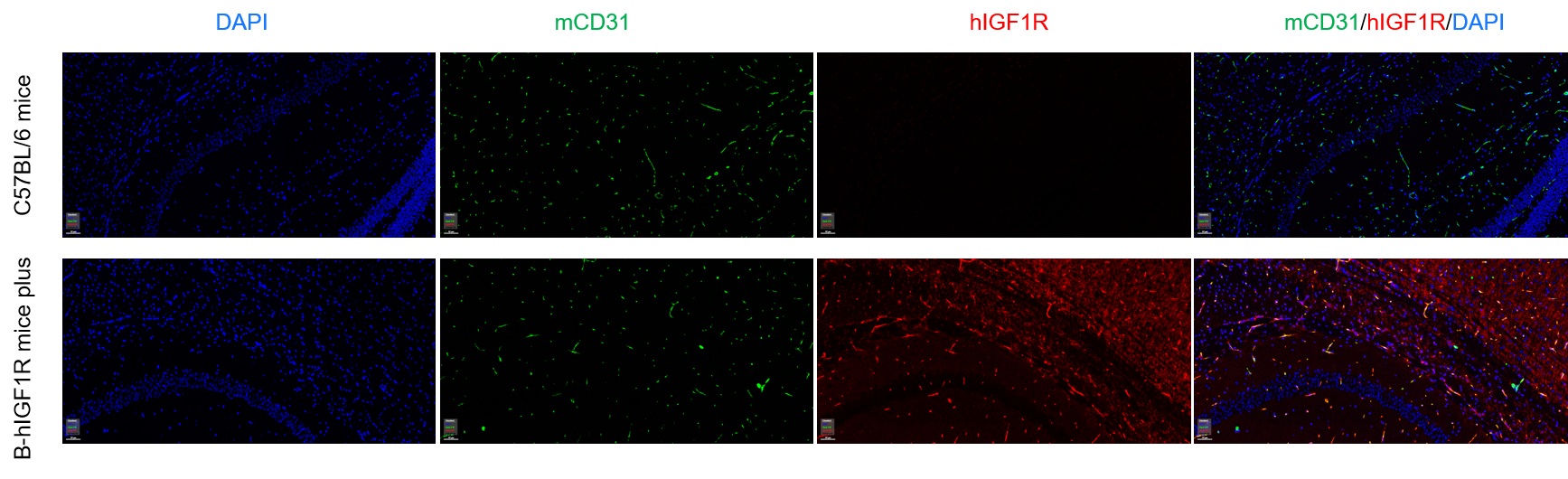
Immunofluorescence analysis of isolated brain micro-vessels from the wild-type C57BL/6 mice and homozygous B-hIGF1R mice plus. Brain were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hIGF1R mice plus (H/H), and analyzed by immunofluorescence with anti-mCD31 (abcam, ab182981) and anti-hIGF1R antibody (abcam, ab263903). Human IGF1R was exclusively detectable in brain micro-vessels in homozygous B-hIGF1R mice plus but not in wild-type mice.
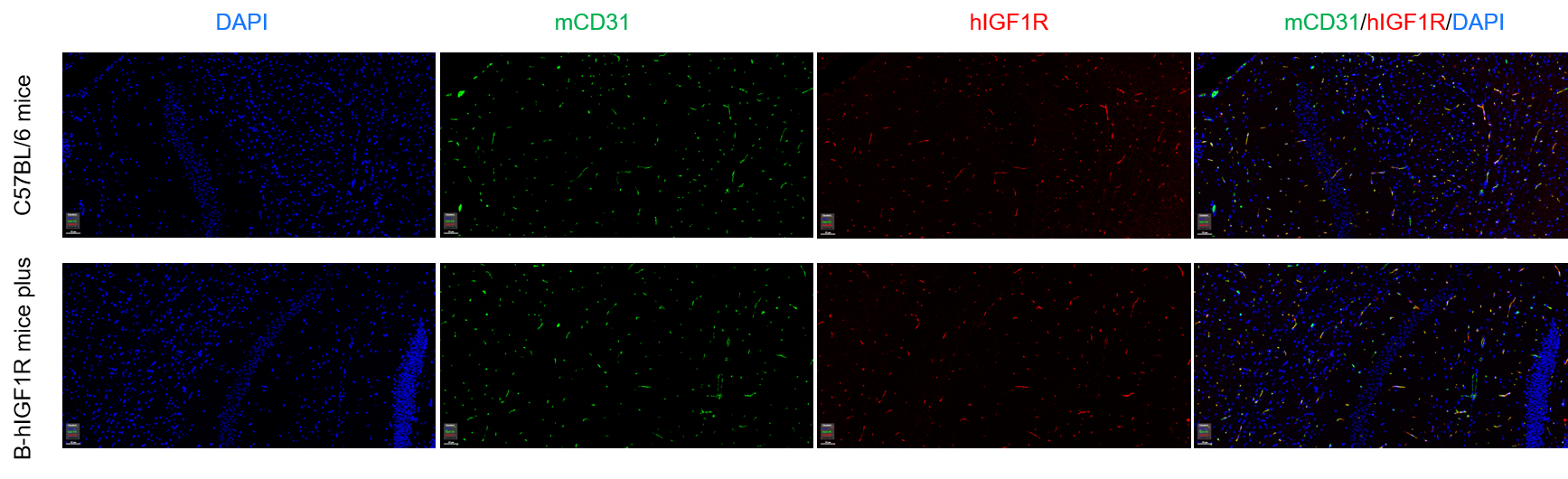
Immunofluorescence analysis of isolated brain micro-vessels from the wild-type C57BL/6 mice and homozygous B-hIGF1R mice plus. Brain were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hIGF1R mice plus (H/H), and analyzed by immunofluorescence with anti-mCD31 (abcam, ab182981) and anti-IGF1R antibody (CST, 3027S). IGF1R was detectable in both wild-type C57BL/6 mice and homozygous B-hIGF1R mice plus, as the antibody was cross-reactive between human and mouse.
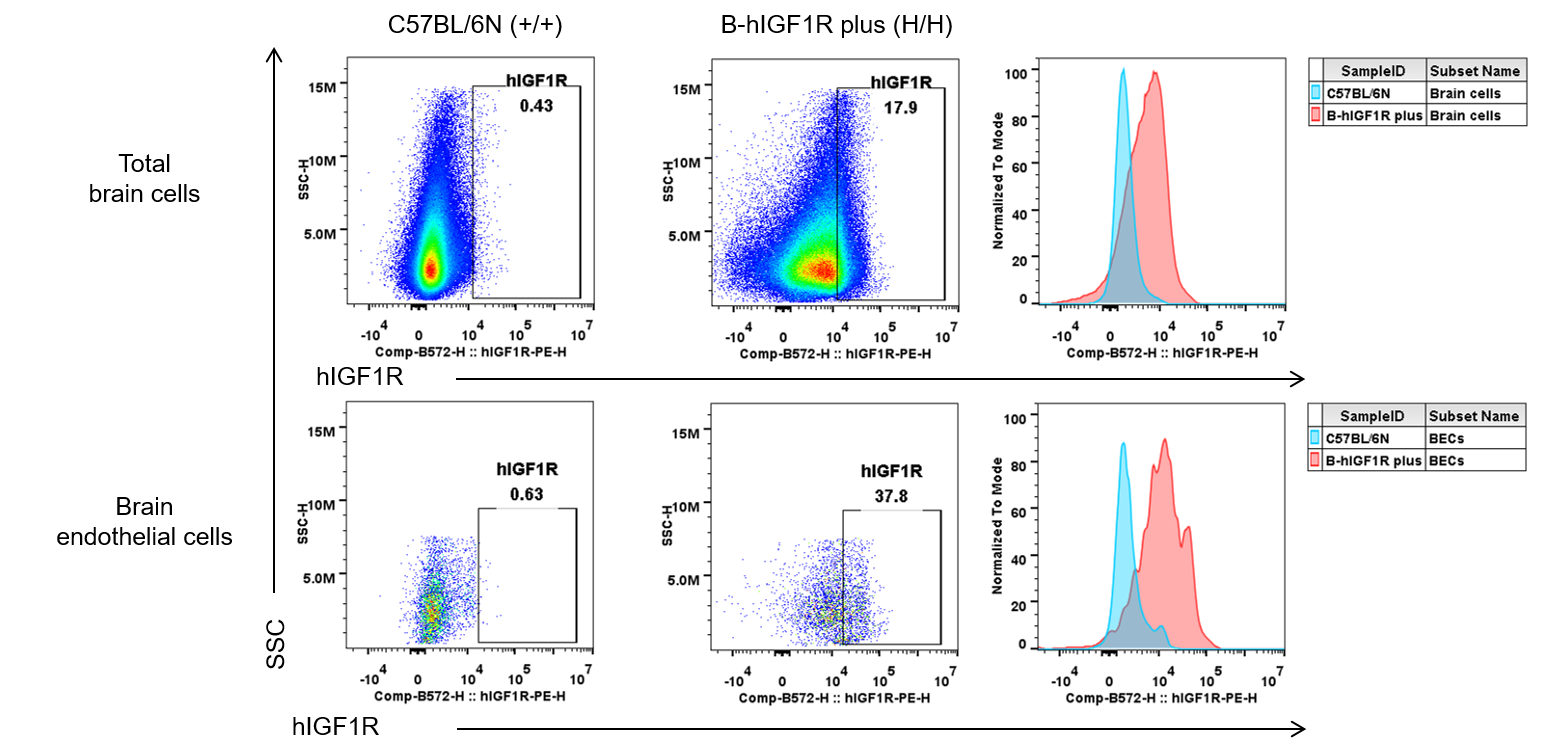
Strain specific IGF1R expression in wild-type C57BL/6N and homozygous B-hIGF1R mice plus by flow cytometry. Brain cells were collected from wild-type C57BL/6N (+/+) and homozygous B-hIGF1R mice plus (H/H) (male, 7-week-old, n=1) and analyzed by flow cytometry with anti-human IGF1R antibody (BioLegend, 351805). Human IGF1R was exclusively detectable in total brain cells and endothelial cells of homozygous B-hIGF1R mice plus.
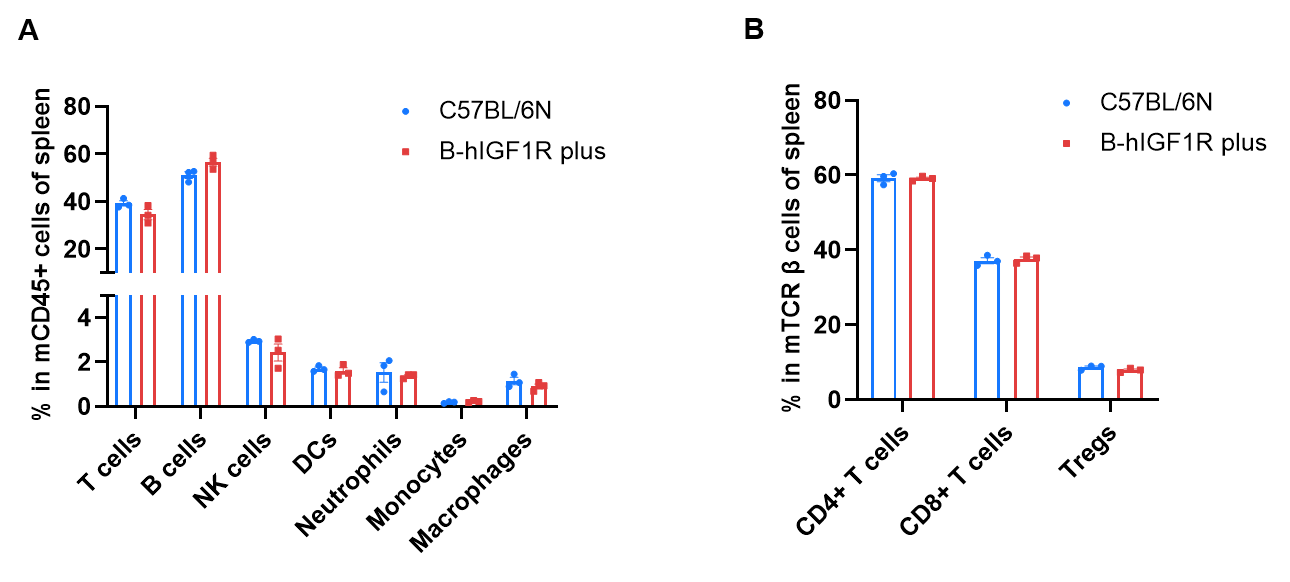
Frequency of leukocyte subpopulations in spleen by flow cytometry. Splenocytes were isolated from wild-type C57BL/6N mice (male, n=3, 7-week-old) and homozygous B-hIGF1R mice plus (male, n=3, 7-week-old). A. Flow cytometry analysis of the splenocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percentages of T cells, B cells, NK cells, dendritic cells, Neutrophils, monocytes, macrophages, CD4+T cells, CD8+T cells and Tregs in B-hIGF1R mice plus were similar to those in C57BL/6 mice. The frequency of leukocyte subpopulations in blood and lymph nodes of B-hIGF1R mice plus were also comparable to wild-type C57BL/6N mice (Data not shown). Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA test. *p < 0.05, **p < 0.01, ***p < 0.001.

Western blot analysis of phospho-IGF1R/AKT protein expression in wild-type C57BL/6N mice and homozygous B-hIGF1R mice plus. Kidney cells were collected from wild-type C57BL/6N mice (+/+), homozygous B-hIGF1R mice (H/H) and homozygous B-hIGF1R mice plus (H/H), and then cultured with Figitumumab (1 μg/mL) or/and recombinant human IGF1 protein (200 ng/mL). Finally, these cells were analyzed by western blot with anti-IGF1R antibody (CST, 3027S), anti-phospho-IGF1R antibody (CST, 3024S) and anti-phospho-AKT antibody (CST, 9271S). IGF1 induces phosphorylation of IGF1R in both wild-type and B-hIGF1R mice plus, and figitumumab-analog effectively suppresses IGF1-induced activation of humanized IGF1R in B-hIGF1R mice plus, whereas phosphorylation of endogenous mouse IGF1R remains unaffected, reflecting the species-restricted binding profile of Figitumumab. M, marker.
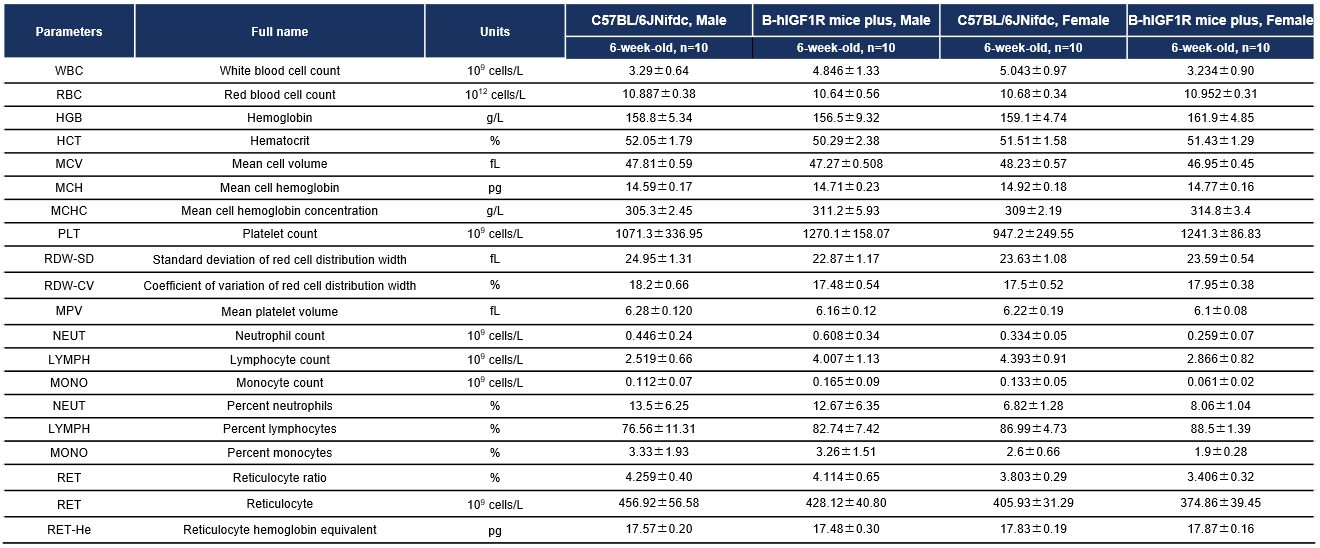
Complete blood count (CBC). Blood from male and female C57BL/6JNifdc and B-hIGF1R mice plus (n=10, 6 week-old) were collected and analyzed for CBC. There was no differences among any measurement between C57BL/6JNifdc and B-hIGF1R mice plus, indicating that humanization of IGF1R does not change blood cell composition and morphology. Values are expressed as mean ± SEM.
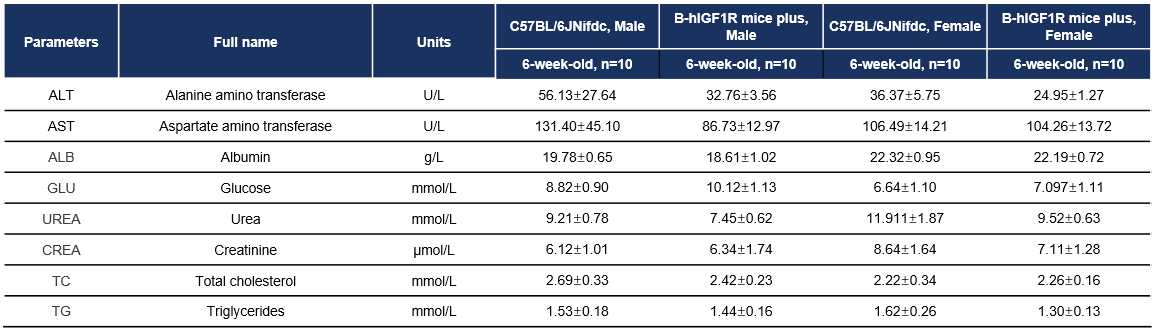
Blood chemistry tests of B-hIGF1R mice plus. Serum from male and female C57BL/6JNifdc and B-hIGF1R mice plus (n=10, 6 week-old) were collected for biochemistry analysis. There was no differences among any measurement between male C57BL/6JNifdc and male B-hIGF1R mice plus, indicating that humanization of IGF1R does not change biochemistry of blood. Values are expressed as mean ± SEM.
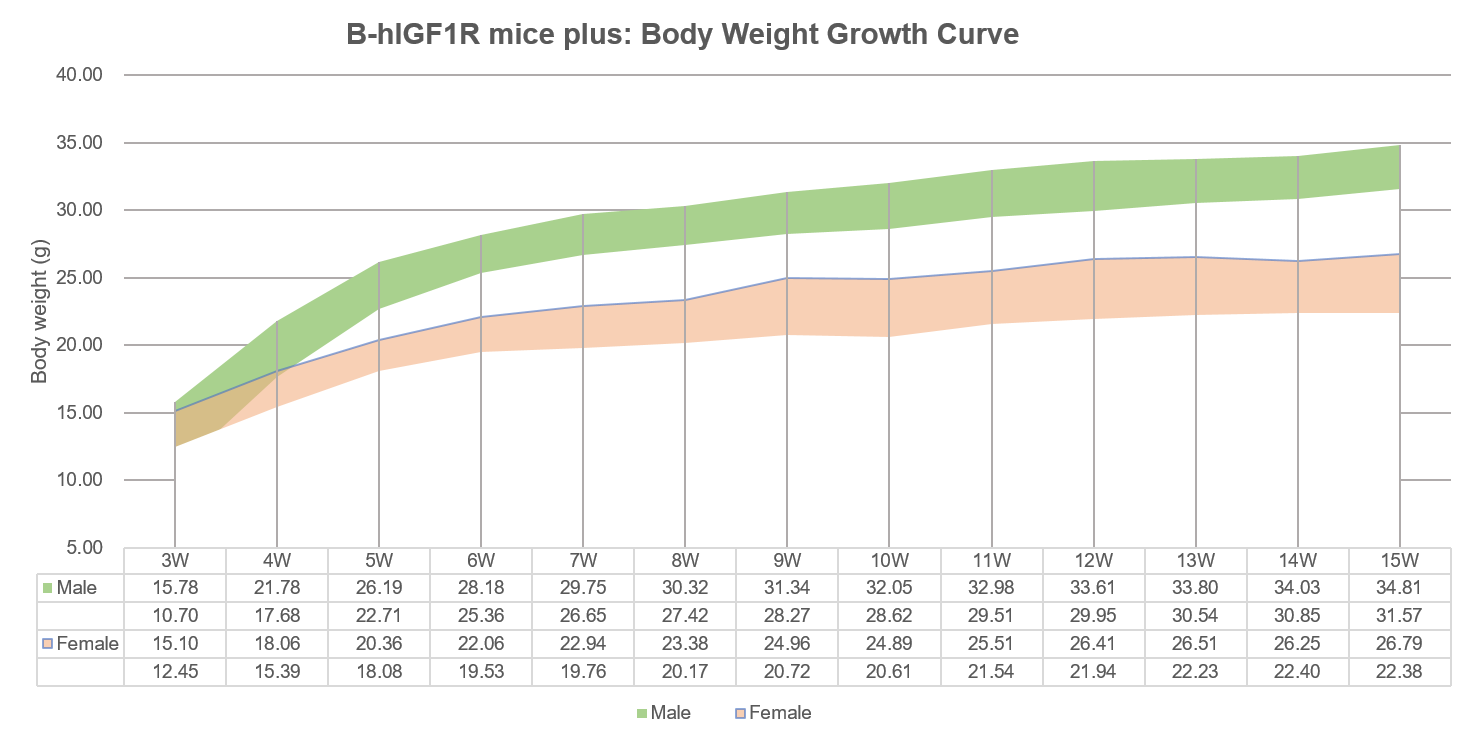
Body weight growth curve of B-hIGF1R mice plus. 10 males and 10 females homozygous B-hIGF1R mice plus were randomly selected and body weight was collected once a week. The minimum and maximum weights of the mice in the table are calculated as the average ± SD. The body weight growth curve is consistent with that of wild-type mice.
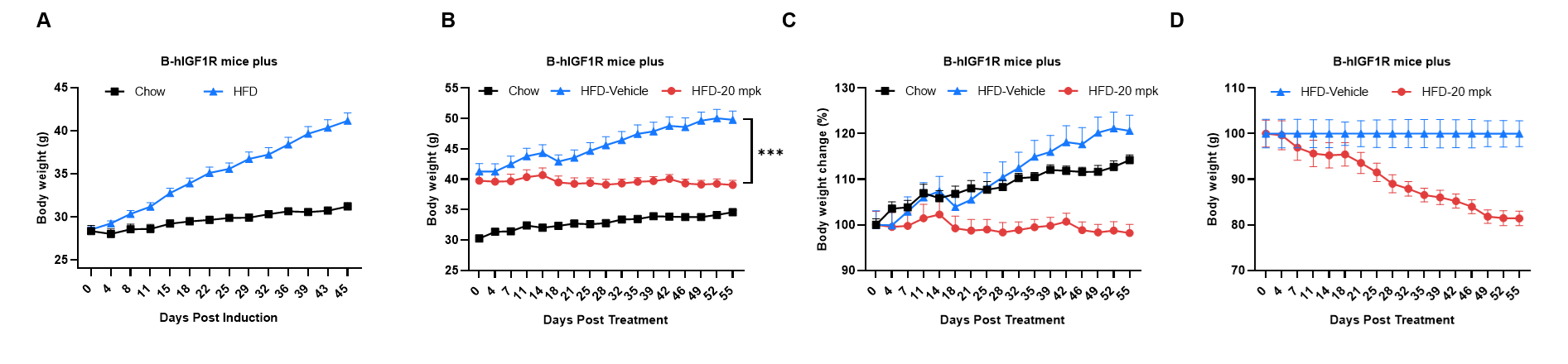
Efficacy study of anti-IGF1R antibody in HFD induced B-hIGF1R mice plus. B-hIGF1R mice plus were fed with a high-fat diet to induce obesity. (A) Body weight change after HFD induction. (B-D) Body weight change after anti-IGF1R antibody (provided by a client) treatment. The anti-IGF1R antibody significantly inhibits HFD-induced body weight gain, as evidenced by lower body weight and reduced body weight change in the treatment group (red) compared to the HFD-Vehicle group (blue). ***p < 0.001. Values are expressed as mean ± SEM.
Note: This experiment is a collaborative validation project with the client.
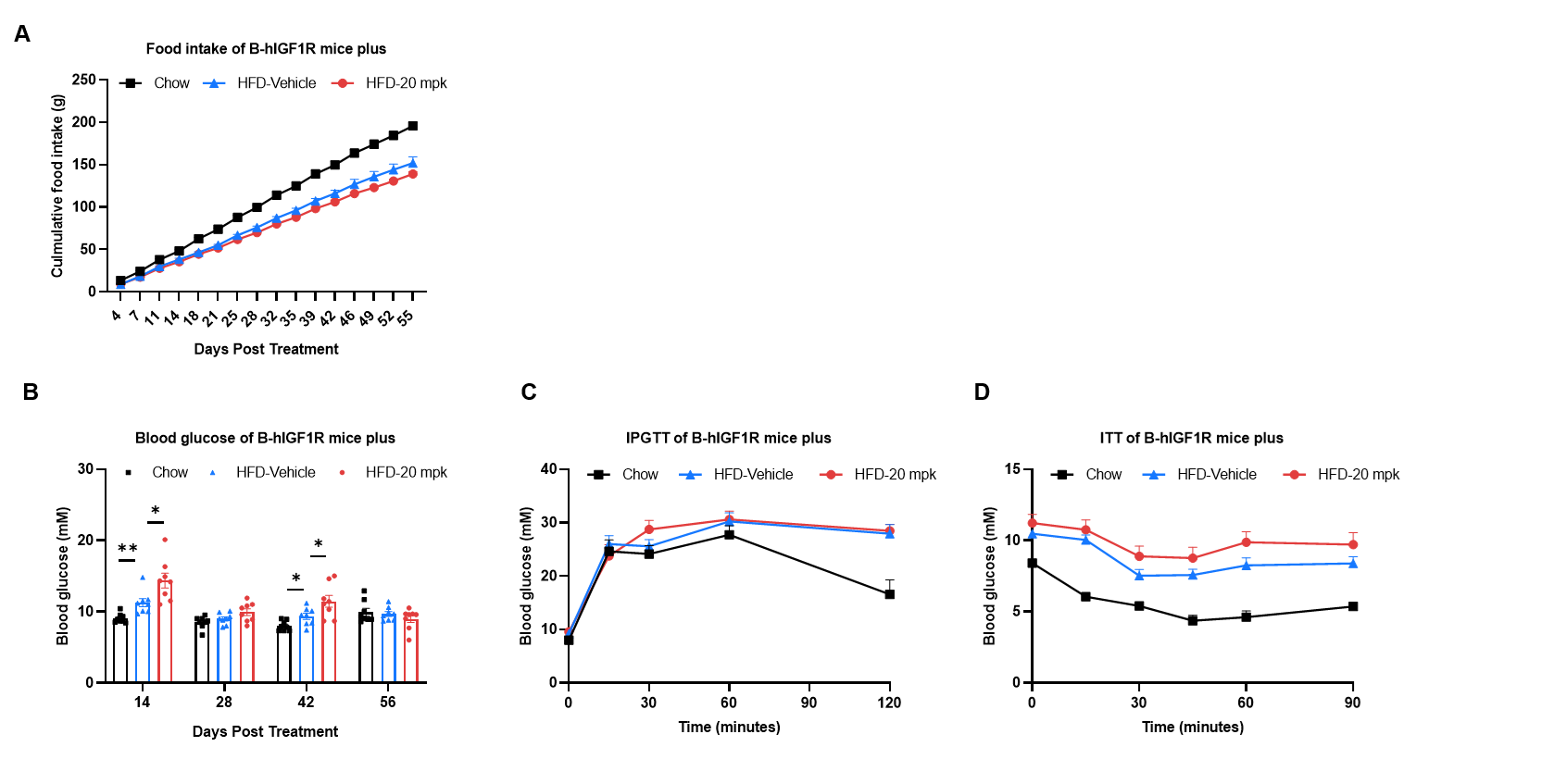
Efficacy study of anti-IGF1R antibody in HFD induced B-hIGF1R mice plus. B-hIGF1R mice plus were fed with a high-fat diet to induce obesity. (A) Effect of anti-IGF1R antibody (provided by a client) on food intake. (B) Blood glucose change after anti-IGF1R antibody treatment. (C) The glucose tolerance test for 15% D-Glucose is administered by intraperitoneal injection (IPGTT). (D) The insulin tolerance test for 0.5 U/kg is administered by intraperitoneal injection (ITT). Values are expressed as mean ± SEM. Significance was determined by the Ordinary one-way ANOVA. *p<0.05, **p<0.01, ***p<0.001.
Note: This experiment is a collaborative validation project with the client.