
Non-disclosure • 112744

| Product name | B-hTSLP/hTSLPR mice plus |
|---|---|
| Catalog number | 112744 |
| Strain name | Non-disclosure |
| Strain background | C57BL/6 |
| NCBI gene ID | 85480,64109,3575 (Human) |
| Aliases | CRL2; TSLPR; CRLF2Y; ILRA; CD127; IL7RA; CDW127; IMD104; sIL-7R; lnc-IL7R; IL7Ralpha; IL-7Ralpha; IL-7R-alpha |
Thymic stromal lymphopoietin (TSLP) is an epithelial-cell–derived cytokine, while TSLP receptor (TSLPR, also known as CRLF2) mediates TSLP signaling in immune cells. Dysregulated TSLP–TSLPR signaling has been implicated in allergic asthma, atopic dermatitis, inflammatory bowel disease (IBD), and autoimmune disorders.
The B-hTSLP/hTSLPR humanized mouse model was generated by replacing murine Tslp and Tslpr genes with their human orthologs. These mice express functional human TSLP and TSLPR, enabling the study of human-specific cytokine signaling and therapeutic interventions. This double humanized system provides a robust platform for preclinical efficacy evaluation, antibody validation in vivo, and autoimmune/inflammatory disease modeling.
Key Advantages
Validation
Applications
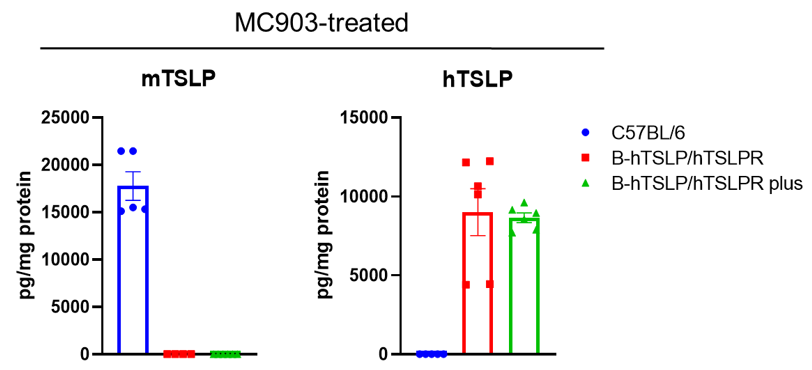
Strain specific TSLP expression analysis in wild-type C57BL/6 mice, homozygous B-hTSLP/hTSLPR mice and B-hTSLP/hTSLPR mice plus by ELISA. Calcipotriol (MC903) was dissolved in ethanol and topically applied on ears of wild-type C57BL/6 mice, homozygous B-hTSLP/hTSLPR mice and B-hTSLP/hTSLPR mice plus for 7 days (male, 8-week-old, n=3). Expression level of mouse and human TSLP in ear grinding supernatant from the three strains of mice were analyzed by ELISA (mouse TSLP: Biolegend, 434107; human TSLP: Biolegend, 434207). Mouse TSLP was only detectable in wild-type C57BL/6 mice. Human TSLP was detectable in homozygous B-hTSLP/hTSLPR mice and B-hTSLP/hTSLPR mice plus but not in wild-type mice.
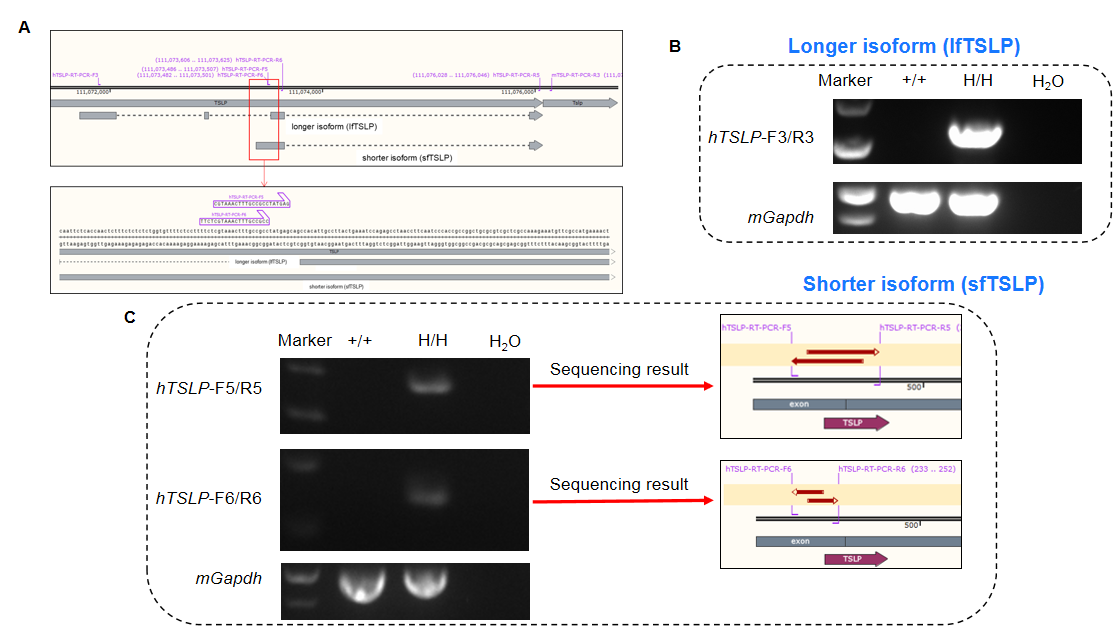
Long and short isoforms of human TSLP were assayed by RT-PCR and Sanger sequencing. Ear tissues were collected from wild-type C57BL/6 (+/+) and homozygous TSLP/TSLPR humanized mice plus (H/H). (A) Isoform-specific primers were designed to detect long (lfTSLP) and short (sfTSLP) human TSLP transcripts. (B) lfTSLP mRNA was detectable in TSLP/TSLPR humanized mice plus, but not in wild-type mice. (C) sfTSLP mRNA was detectable in TSLP/TSLPR humanized mice plus, but not in wild-type mice. Sequencing of sfTSLP PCR products confirmed identity to database reference sequences.
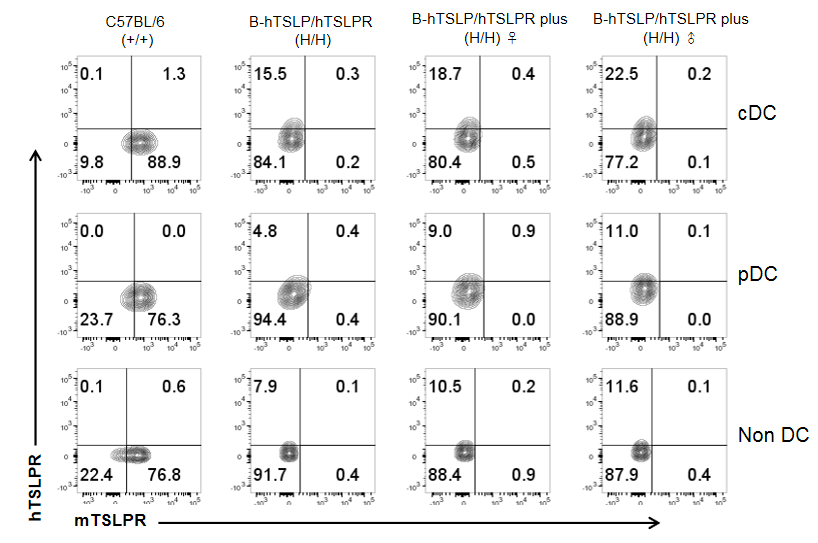
Mouse and human TSLPR protein expression analysis in splenocytes. Splenocytes from wild-type C57BL/6, homozygous TSLP/TSLPR humanized mice, and TSLP/TSLPR humanized mice plus were stained with species-specific anti-TSLPR antibodies. Mouse TSLPR was detected on cDC, pDC, and non-DCs in wild-type mice, but not in TSLPR-humanized strains. Human TSLPR was detected on cDC, pDC, and non-DCs in TSLP/TSLPR humanized mice and TSLP/TSLPR humanized mice plus, but not in wild-type mice. Values are mean ± SEM.
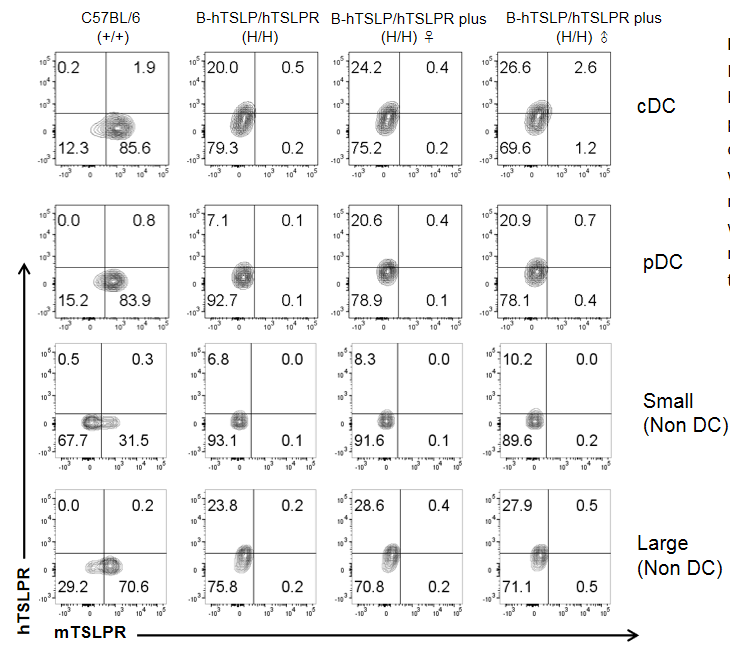
Mouse and human TSLPR protein expression analysis in bone marrow. Bone marrow cells from wild-type C57BL/6, homozygous TSLP/TSLPR humanized mice, and TSLP/TSLPR humanized mice plus were analyzed by flow cytometry. Mouse TSLPR was detectable on cDC, pDC, and non-DCs in wild-type mice, but absent in TSLPR-humanized strains. Human TSLPR was detectable on cDC, pDC, and non-DCs in both humanized strains, but absent in wild-type mice. Values are mean ± SEM.
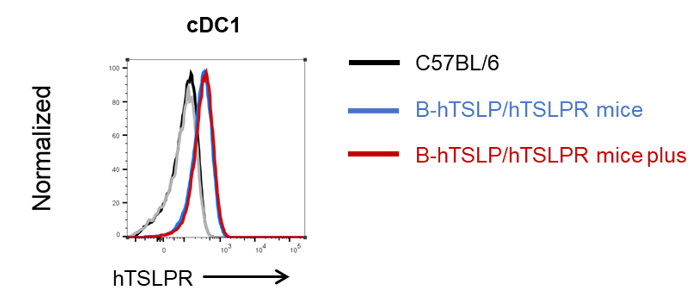
Human TSLPR expression analysis in cDC1 from bone marrow. Bone marrow from wild-type C57BL/6, homozygous TSLP/TSLPR humanized mice, and TSLP/TSLPR humanized mice plus was analyzed by flow cytometry with species-specific antibodies. Human TSLPR was highly expressed on cDC1 in both humanized strains.
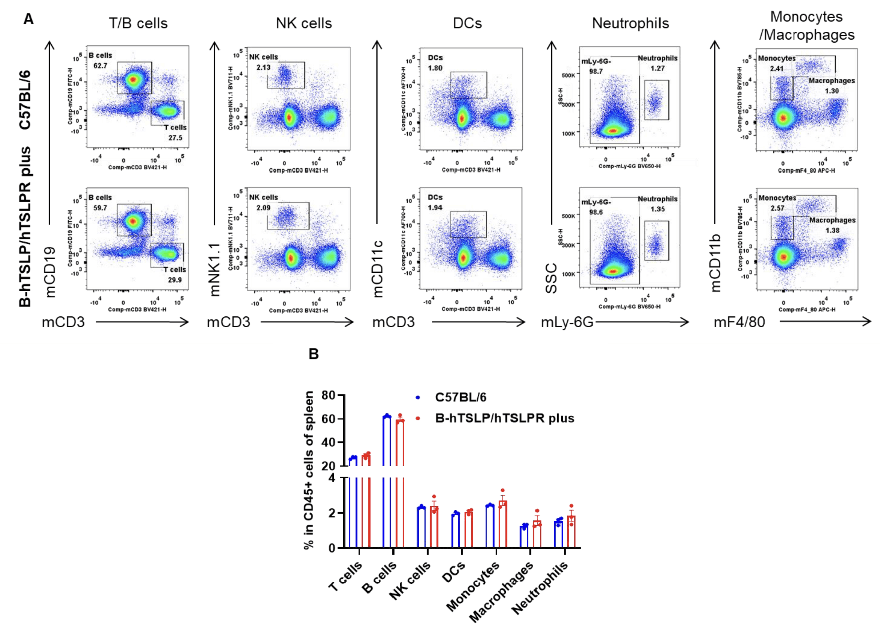
Analysis of spleen leukocyte subpopulations by flow cytometry. Splenocytes from female C57BL/6 and TSLP/TSLPR humanized mice plus (n=3, 9-week-old) were profiled by flow cytometry. A) Representative FACS plots: single, live CD45+ cells gated for downstream analysis. B) Quantification: percentages of T cells, B cells, NK cells, DCs, neutrophils, monocytes, and macrophages in TSLP/TSLPR humanized mice plus were similar to C57BL/6, demonstrating that TSLP, TSLPR, and IL7R humanization does not alter overall development, differentiation, or distribution of splenic leukocytes. Values are mean ± SEM.
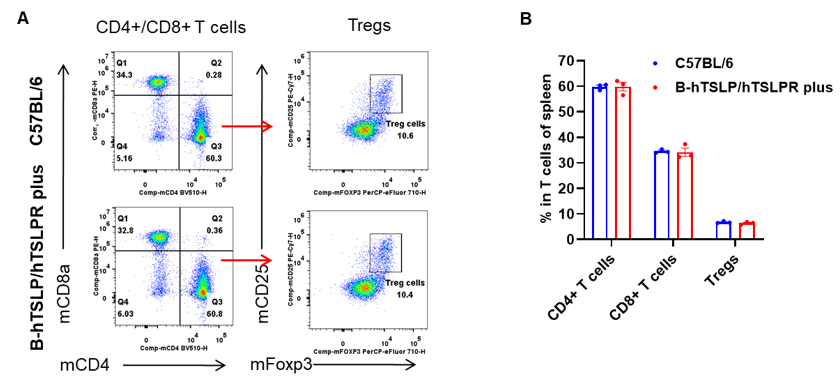
Analysis of T cell subpopulations by flow cytometry. Spleen T-cell subsets were analyzed in female C57BL/6 and TSLP/TSLPR humanized mice plus (n=3, 9-week-old). A) Representative FACS plots: CD45+ → CD3+ gating strategy. B) CD4+ T cells, CD8+ T cells, and Tregs percentages were similar between strains, indicating humanization does not impact T-cell development in spleen. Values are mean ± SEM.
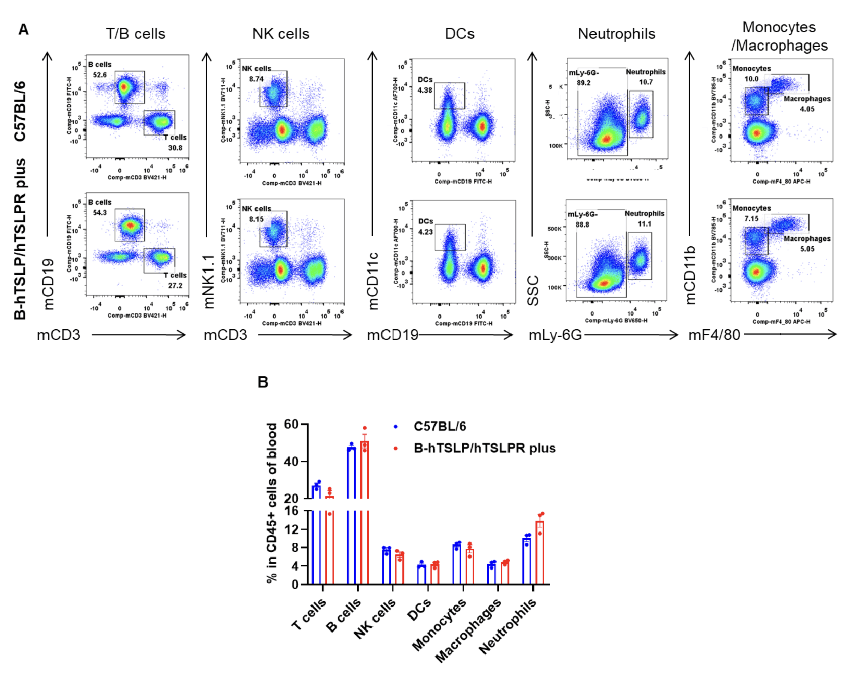
Analysis of blood leukocyte subpopulations by flow cytometry. Blood from female C57BL/6 and TSLP/TSLPR humanized mice plus (n=3, 9-week-old) was analyzed by flow cytometry. A) Representative FACS plots: single, live CD45+ cells gated for analysis. B) T cells, B cells, NK cells, dendritic cells, neutrophils, monocytes, macrophages showed similar percentages across strains, indicating no perturbation of peripheral leukocyte distribution by humanization. Values are mean ± SEM.
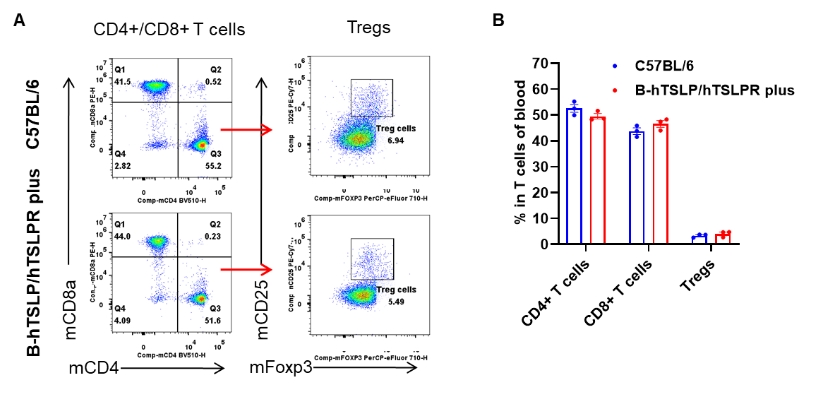
Analysis of blood T cell subpopulations by flow cytometry. Blood samples were isolated from female C57BL/6 mice and TSLP/TSLPR humanized mice plus (n=3, 9-week-old). Flowcytometry analysis of the blood was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percentages of CD4+ T cells, CD8+ T cells, and Tregs in homozygous B-hTSLP/hTSLPR mice plus were comparable to those in wild-type C57BL/6 mice. These results demonstrate that humanization of TSLP and TSLPR does not alter the overall development, differentiation, or distribution of blood T cell subsets in TSLP/TSLPR humanized mice plus. Data are expressed as mean ± SEM.
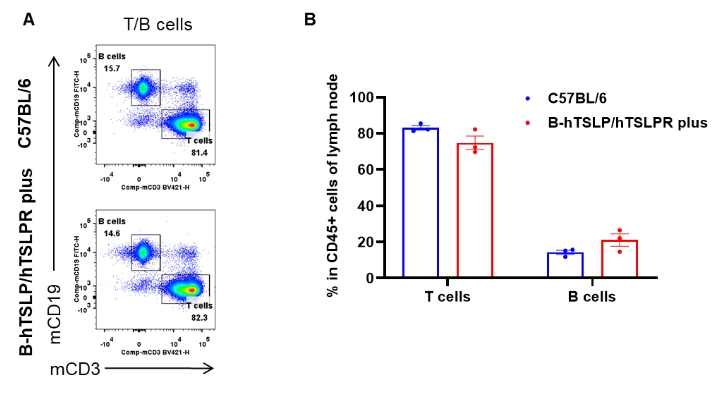
Lymph node leukocyte subpopulations in TSLP/TSLPR humanized and wild-type mice. Lymph nodes were isolated from female C57BL/6 mice and TSLP/TSLPR humanized mice plus (n=3, 9-week-old). Flow cytometry was performed to assess lymph node leukocyte subpopulations. (A) Representative FACS plots: single live cells were gated for the CD45+ population and further analyzed. (B) Results of FACS analysis: the percentages of T cells and B cells in homozygous TSLP/TSLPR humanized mice plus were comparable to those in wild-type C57BL/6 mice. These findings indicate that humanization of TSLP, TSLPR, and IL7R does not alter the development, differentiation, or distribution of T and B cell subsets in lymph nodes of TSLP humanized mice. Values are expressed as mean ± SEM.
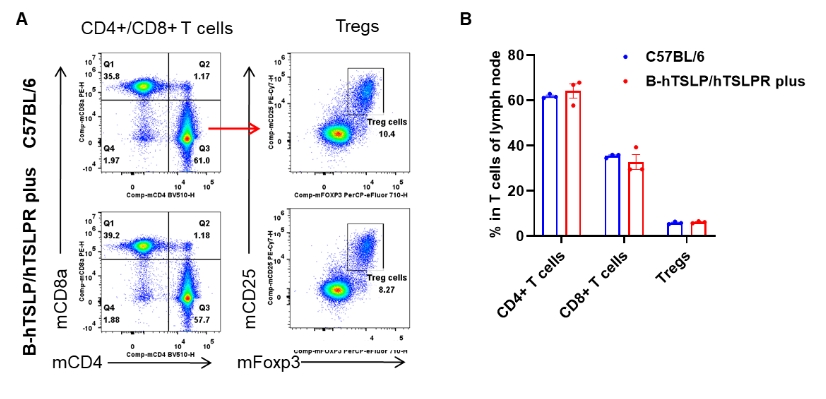
Lymph node T cell subsets in TSLP/TSLPR humanized vs. wild-type mice. Lymph nodes were isolated from female C57BL/6 mice and TSLP/TSLPR humanized mice plus (n=3, 9-week-old). Flow cytometry was conducted to assess lymph node T cell subpopulations. (A) Representative FACS plots: single live CD45+ cells were gated for the CD3+ T cell population and analyzed further. (B) Results of FACS analysis: the percentages of CD4+ T cells, CD8+ T cells, and Tregs in homozygous TSLP/TSLPR humanized mice plus were comparable to those in wild-type C57BL/6 mice. These results demonstrate that humanization of TSLP, TSLPR, and IL7R does not alter the overall development, differentiation, or distribution of T cell subsets in lymph nodes of TSLP humanized mice. Values are expressed as mean ± SEM.
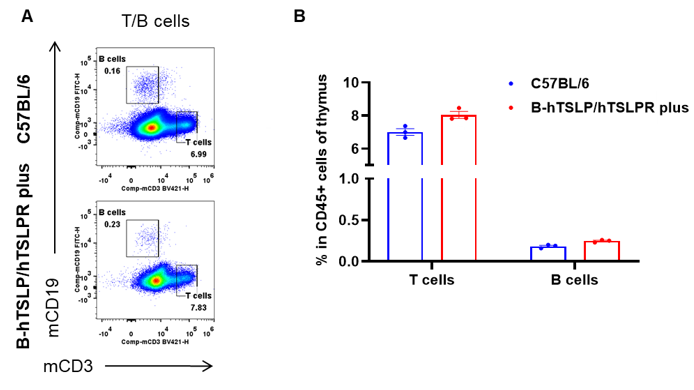
Flow Cytometric Analysis of Thymic Leukocyte Subpopulations in TSLP/TSLPR Humanized Mice. Thymuses were isolated from female C57BL/6 mice and TSLP/TSLPR humanized mice plus (n=3, 9-week-old). Flow cytometry was performed to assess thymic leukocyte subpopulations. A. Representative FACS plots: single live cells were gated for the CD45+ population and analyzed further. B. Results of FACS analysis: the percentages of T cells and B cells in homozygous TSLP/TSLPR humanized mice plus were comparable to those in wild-type C57BL/6 mice. These findings indicate that humanization of TSLP, TSLPR, and IL7R does not affect the overall development, differentiation, or distribution of thymic leukocyte subsets in TSLP humanized mice. Values are expressed as mean ± SEM.
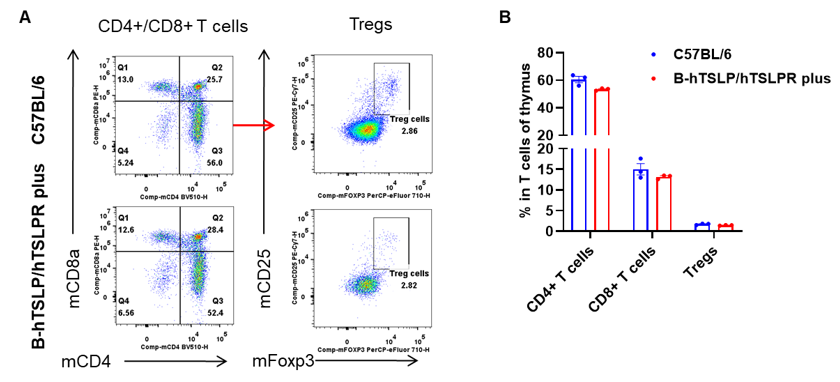
Flow Cytometric Analysis of Thymic T-Cell Subsets in TSLP/TSLPR Humanized Mice. Thymuses were isolated from female C57BL/6 mice and TSLP/TSLPR humanized mice plus (n=3, 9-week-old). Flow cytometry was performed to assess thymic T cell subpopulations. A. Representative FACS plots: single live CD45+ cells were gated for the CD3+ T cell population and analyzed further. B. Results of FACS analysis: the percentages of CD4+ T cells, CD8+ T cells, and Tregs in homozygous TSLP/TSLPR humanized mice plus were comparable to those in wild-type C57BL/6 mice. These results demonstrate that humanization of TSLP, TSLPR, and IL7R does not alter the development, differentiation, or distribution of thymic T cell subsets in TSLP humanized mice. Values are expressed as mean ± SEM.
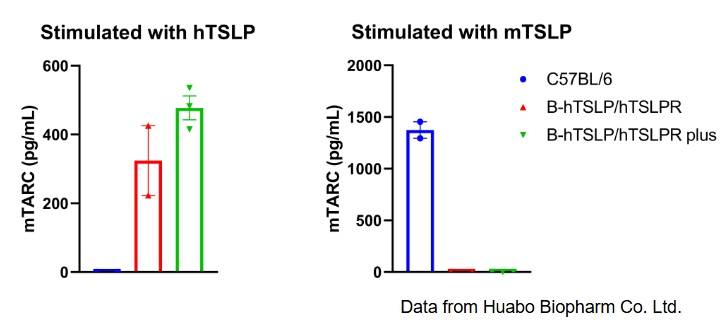
Mouse TARC production was analyzed following stimulation with human TSLP or mouse TSLP in wild-type C57BL/6 mice, homozygous TSLP/TSLPR humanized mice, and TSLP/TSLPR humanized mice plus. Dendritic cells (DCs) were generated from bone marrow using FLT3L and subsequently stimulated with human TSLP or mouse TSLP in vitro. Supernatant concentrations of mouse TARC were measured by ELISA. In homozygous TSLP/TSLPR humanized mice and TSLP/TSLPR humanized mice plus, human TSLP successfully induced mouse TARC secretion, whereas mouse TSLP did not. In contrast, in wild-type C57BL/6 mice, mouse TSLP induced TARC, while human TSLP did not. The level of mouse TARC in TSLP/TSLPR humanized mice plus was higher than in homozygous TSLP/TSLPR humanized mice, confirming stronger responsiveness. These results demonstrate that TSLP–TSLPR signaling is species-specific, with no cross-reactivity between mouse and human. Importantly, human TSLP activates dendritic cells only in TSLP/TSLPR humanized mice, validating the model for antibody efficacy testing and translational immunology research.
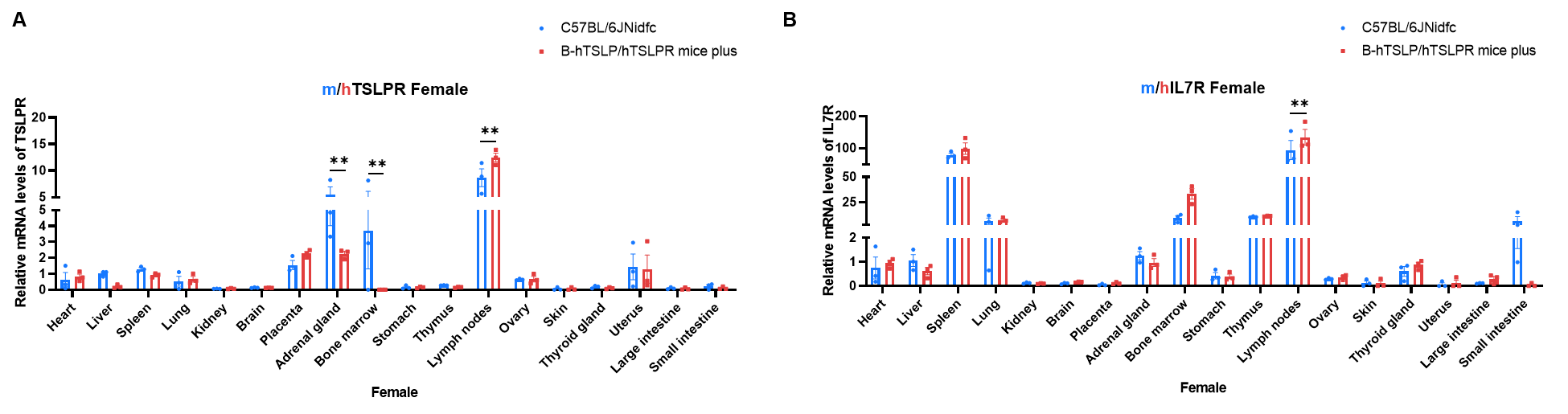
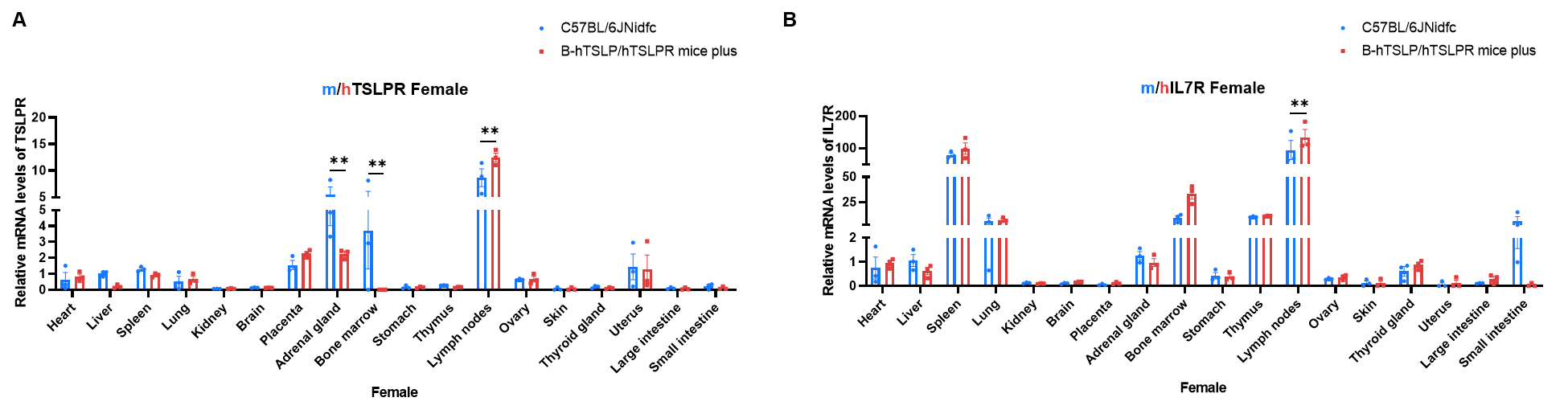
Strain specific analysis of TSLPR and IL7R expression in wild-type C57BL/6JNidfc mice and homozygous B-hTSLP/hTSLPR mice plus by RT-qPCR. Various organizations RNA were isolated from wild-type C57BL/6JNidfc mice (+/+) (n=3, female, 8-week-old) and homozygous B-hTSLP/hTSLPR mice plus (n=3, female, 8-week-old), then cDNA libraries were synthesized by reverse transcription, followed by real-time quantitative PCR with TSLPR and IL7R primers. The mRNA levels of TSLPR and IL7R were in detectable in B-hTSLP/hTSLPR mice plus and wild-type C57BL/6JNidfc mice with Applied Biosystems SYBR. The mRNA levels of TSLPR and IL7R were highly expressed in various immune organs. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***p < 0.001. Normalize to C57BL/6JNidfc-Liver.
Strain specific analysis of TSLPR and IL7R expression in wild-type C57BL/6JNidfc mice and homozygous B-hTSLP/hTSLPR mice plus by RT-qPCR. Various organizations RNA were isolated from wild-type C57BL/6JNidfc mice (+/+) (n=3, male, 8-week-old) and homozygous B-hTSLP/hTSLPR mice plus (n=3, male, 8-week-old), then cDNA libraries were synthesized by reverse transcription, followed by real-time quantitative PCR with TSLPR and IL7R primers. The mRNA levels of TSLPR and IL7R were in detectable in B-hTSLP/hTSLPR mice plus and wild-type C57BL/6JNidfc mice with Applied Biosystems SYBR. The mRNA levels of TSLPR was highly expressed in testis. The mRNA levels of IL7R was highly expressed in various immune organs. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***p < 0.001. Normalize to C57BL/6JNidfc-Liver.
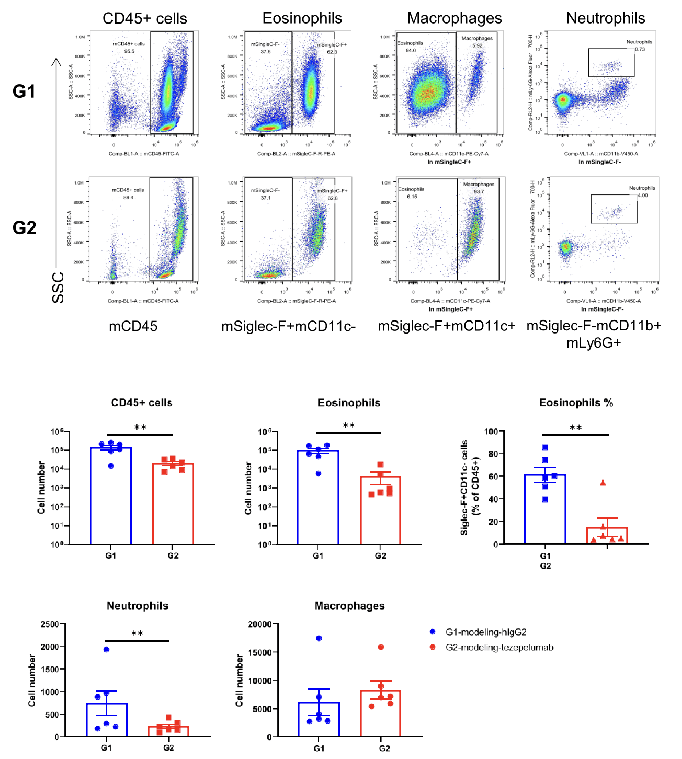
An asthma model was induced in TSLP/TSLPR humanized mice plus and treated with an anti-humanized TSLP antibody (tezepelumab, synthesized in-house) or isotype control antibody. Bronchoalveolar lavage fluid (BALF) was collected at the end of the study to evaluate inflammatory cell infiltration in lung tissue. Results showed that the percentages of CD45+ cells, eosinophils, and neutrophils in the group treated with anti-humanized TSLP antibody (G2) were significantly reduced compared with the group treated with isotype antibody (G1). Values are expressed as mean ± SEM. These findings confirm that TSLP/TSLPR humanized mice are responsive to anti-humanized TSLP therapy, making them a valuable preclinical model for asthma drug development and antibody validation in vivo.
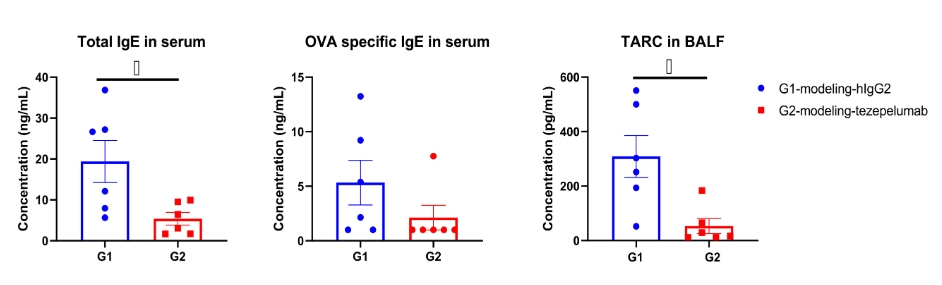
In a mouse asthma model using TSLP/TSLPR humanized mice plus, treatment with an anti-humanized TSLP antibody (tezepelumab, synthesized in-house) significantly reduced OVA-specific IgE in serum and TARC in bronchoalveolar lavage fluid (BALF). Serum was collected at the study endpoint, and both IgE and TARC levels were measured by ELISA. Results demonstrated that levels of OVA-specific IgE and TARC were lower in the antibody-treated group compared with untreated mice. Values are expressed as mean ± SEM.
TARC: thymic and activation-regulated chemokine, also known as CCL17 (C-C motif chemokine ligand 17). These findings confirm that TSLP/TSLPR humanized mice provide a robust preclinical asthma model for evaluating the efficacy of anti-TSLP biologics.
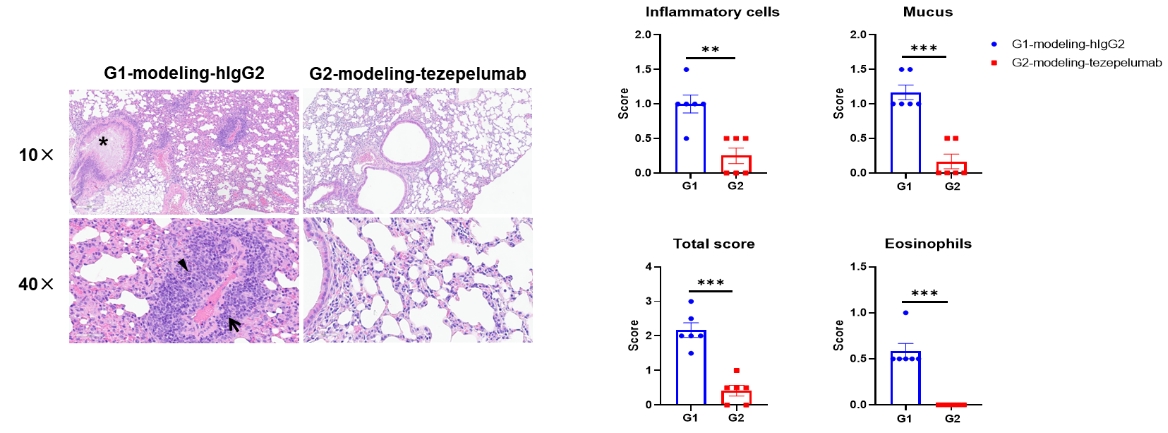
Lung tissues were collected at the study endpoint from TSLP/TSLPR humanized mice plus subjected to an asthma-like model and analyzed by H&E staining.
Compared to the untreated group (G1), mice treated with anti-humanized TSLP antibody (tezepelumab, synthesized in-house) exhibited a significant reduction in inflammatory cell infiltration and mucus secretion in lung tissue.
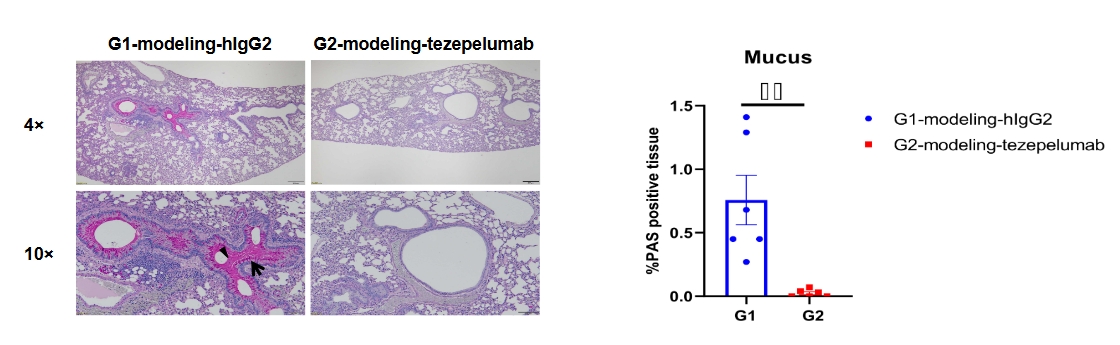
Lung tissues were collected at the study endpoint from TSLP/TSLPR humanized mice plus subjected to an asthma-like model and analyzed by periodic acid-Schiff (PAS) staining. Compared to the untreated group (G1), mice treated with anti-humanized TSLP antibody (tezepelumab, synthesized in-house) exhibited a marked reduction in mucus secretion in lung tissue.
Annotations: Arrow: goblet cells Triangle: mucus. Values are expressed as mean ± SEM. These findings further demonstrate that TSLP/TSLPR humanized mice plus are an effective preclinical model for asthma research, enabling the in vivo evaluation of anti-TSLP antibodies and other therapeutics targeting mucus overproduction and airway inflammation.
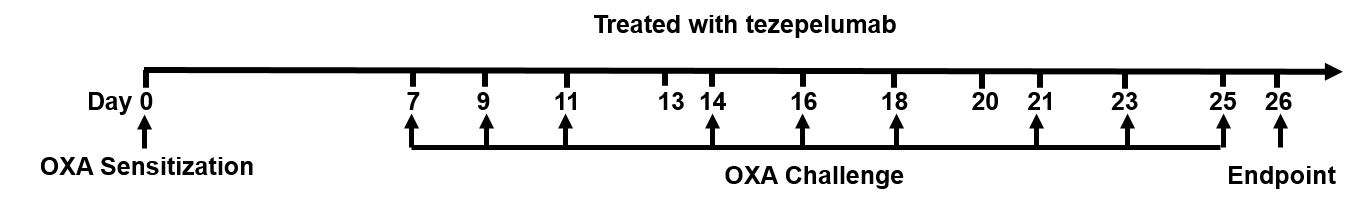
Experimental schedule for Induction of atopic dermatitis (AD)-like skin lesions and in vivo efficacy of anti-human TSLP antibody in B-hTSLP/hTSLPR mice plus. OXA was applied to ear skin of mice on day 0, and then challenge to the same site of skin nine times from days 7 to 25. Anti-human TSLP antibody tezepelumab (in house) was administered by intraperitoneal injection (n = 6). OXA: oxazolone.
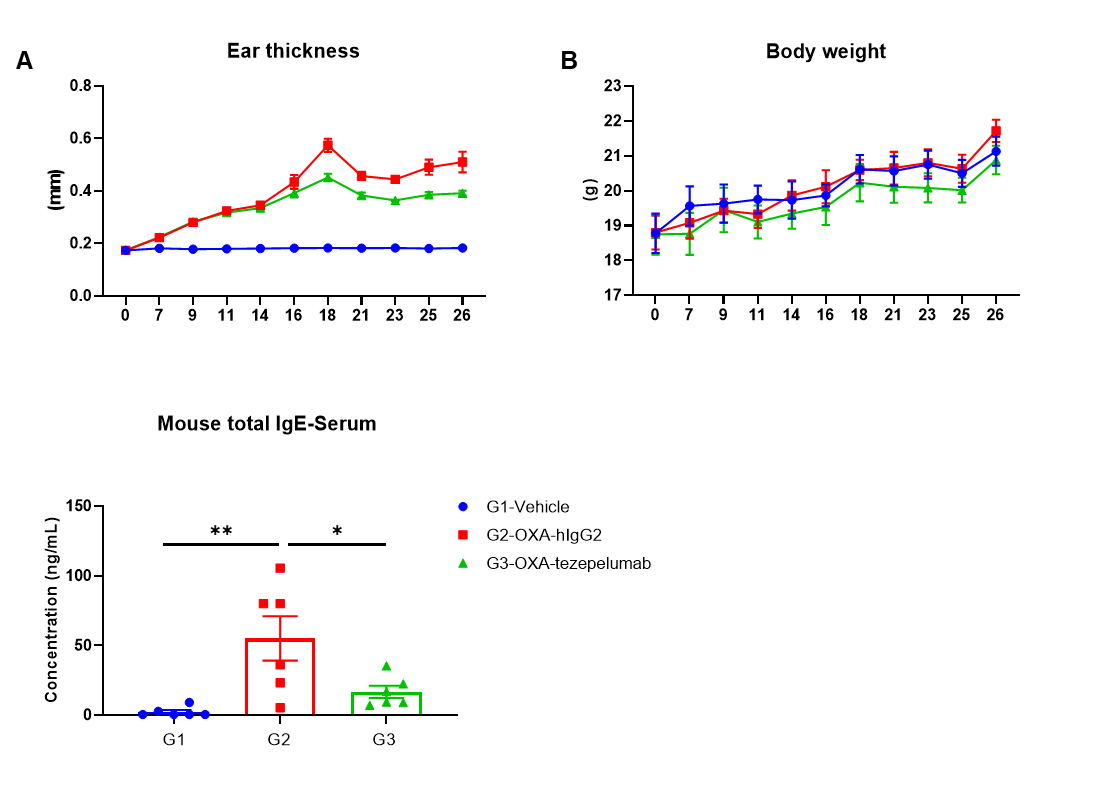
Efficacy of anti-human TSLP antibody in B-hTSLP/hTSLPR mice plus. Mice in each group were treated with anti-hTSLP antibody tezepelumab (in house). (A) Statistical analysis of ear thickness in each group. Epidermis of ear began to desquamate from day 18. So the ear thicknesses were decreased from day 18 as shown in figure. (B) Body weight changes during the treatment. (C) Total IgE levels in serum. Serum was collected on day 26 and total IgE levels were measured by ELISA. (n = 6). Values are expressed as mean ± SEM.
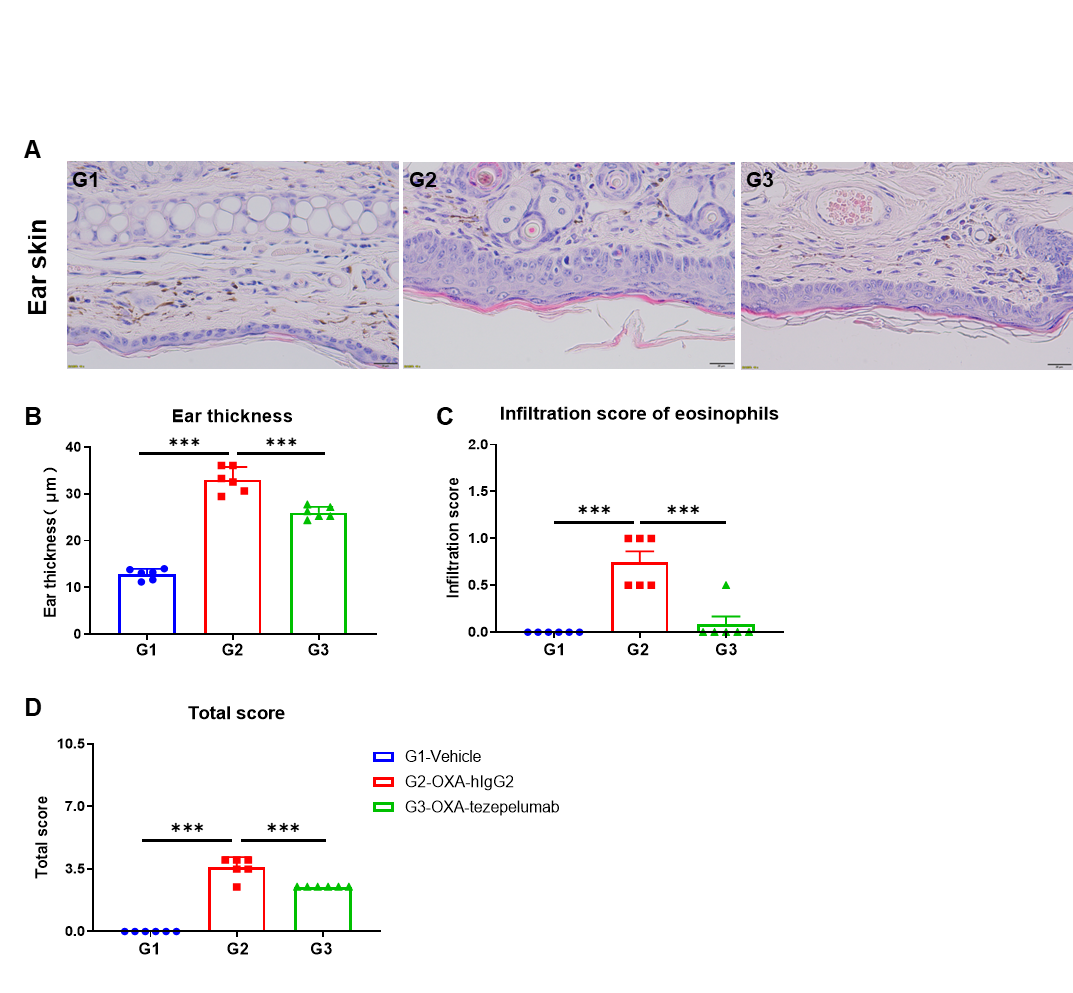
Effects of anti-human TSLP antibody on ear skin of the AD mouse model. (A) Hematoxylin and eosin (H&E) staining. (B) Thickness of ear epidermal skin. (C) Score of eosinophils infiltrated in ear epidermal skin. (D) Total score of ear epidermal skin. Ear thickness and infiltration scores of eosinophils in ear skin of the groups treated with Tezepelumab (in house) were decreased significantly compared to that in the isotype control, demonstrating that the B-hTSLP/hTSLPR mice plus provide a powerful preclinical model for in vivo evaluation of anti-human TSLP antibodies. Infiltration score of eosinophils: 1=slight; 2=mild; 3=moderate; 4=severe. The content of the pathology total score evaluation includes the following aspects: epidermal hyperplasia in skin, erosion/crusting, hyperkeratosis and parakeratosis; inflammatory cell infiltration in dermis and subcutaneous. AD: Atopic dermatitis.
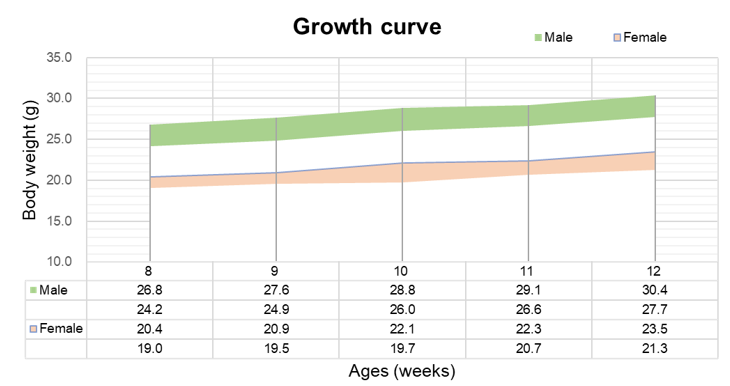
Eight-week-old TSLP/TSLPR humanized mice plus were grouped by sex (10 males and 10 females, respectively). Body weight was measured weekly on the same day for 12 consecutive weeks. The lowest and highest values of body weight in the table were calculated from mean ± SD. The growth curve conformed to a normal distribution, with the probability of random error falling within ± SD estimated at 68%.
These results confirm that TSLP/TSLPR humanized mice plus display normal growth and physiological development, supporting their suitability for long-term pharmacology, toxicology, and antibody validation studies.
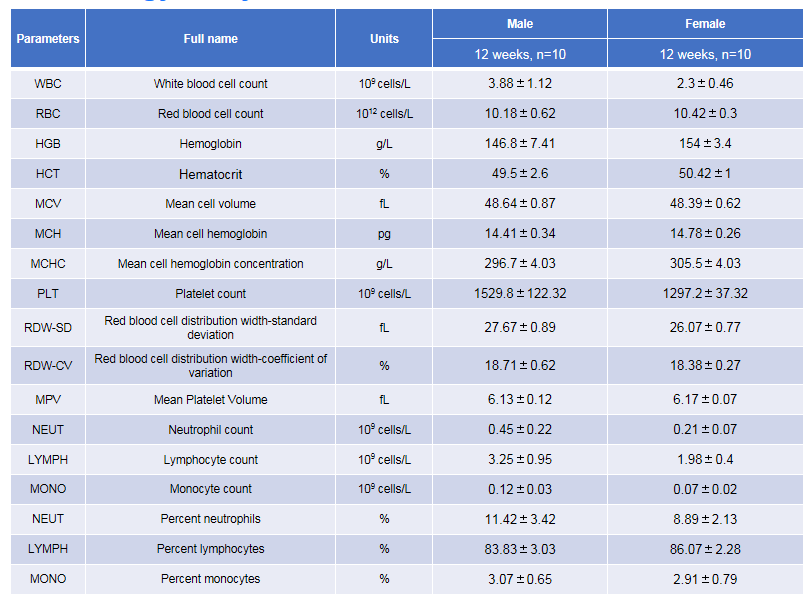
Complete blood count (CBC) was performed in TSLP/TSLPR humanized mice plus. Values are presented as mean ± SD.
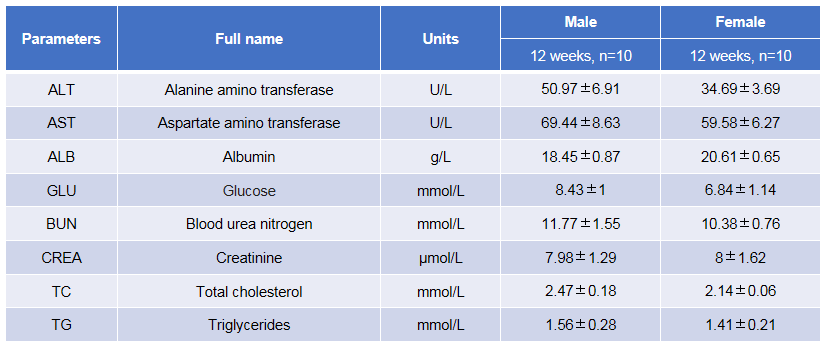
Blood biochemical tests were performed in TSLP/TSLPR humanized mice plus. Values are expressed as mean ± SD.
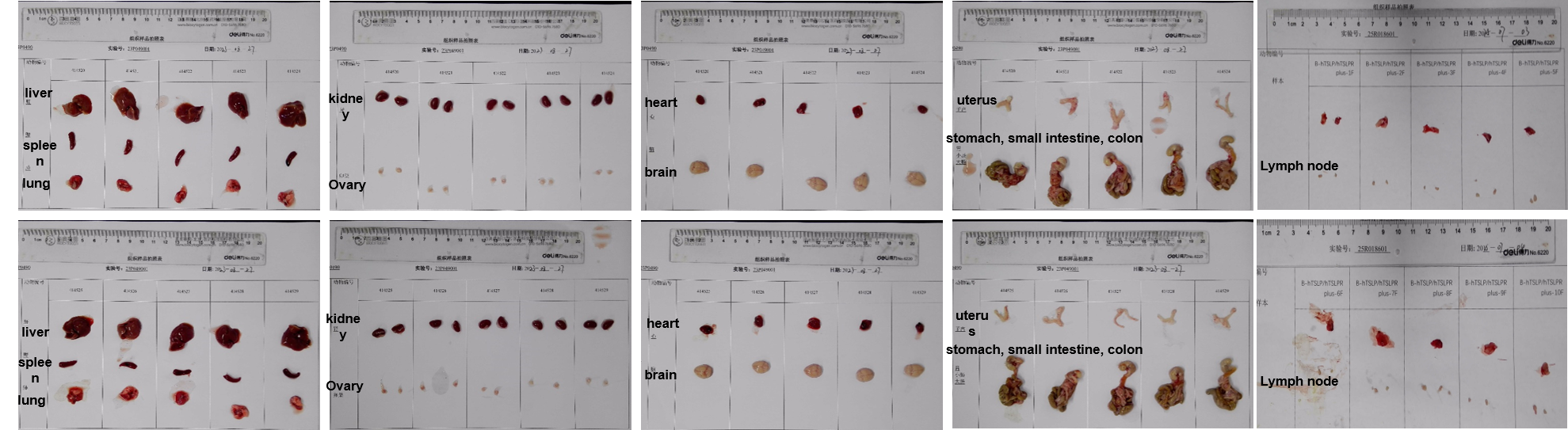
The organs of female B-hTSLP/hTSLPR mice plus (12-week-old, n=10).
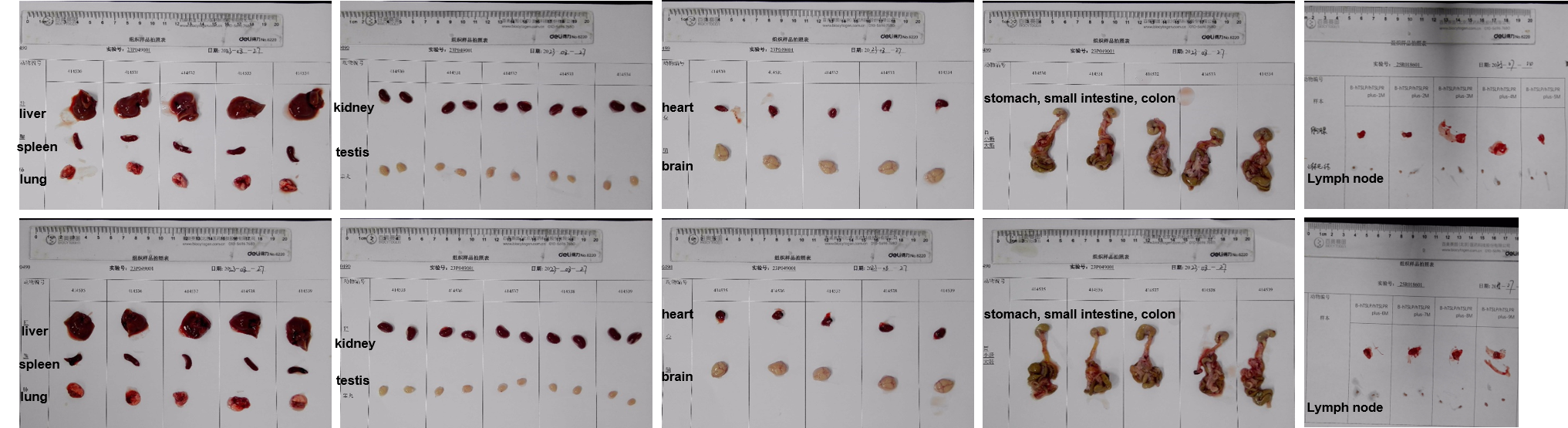
The organs of male B-hTSLP/hTSLPR mice plus (12-week-old, n=10).
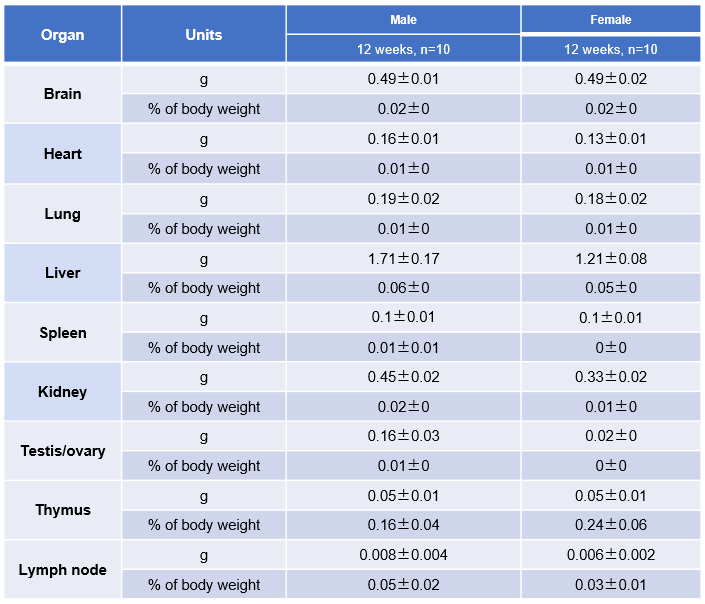
Average weight of the main organs of B-hTSLP/hTSLPR mice plus.
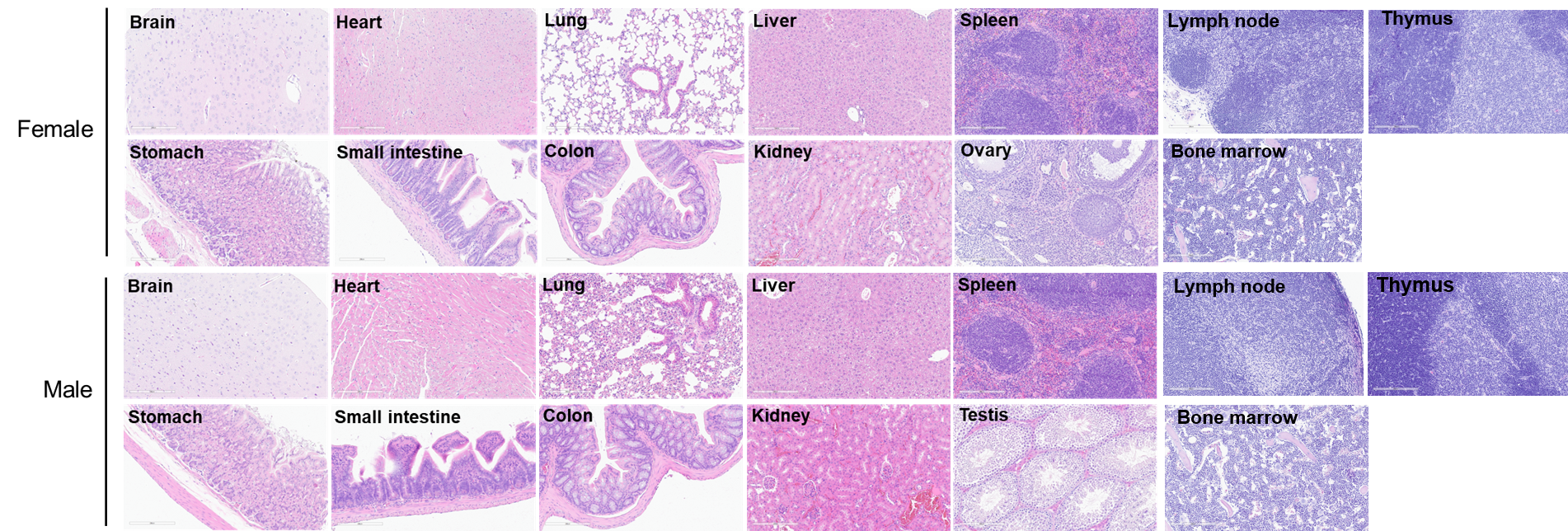
Histopathological analysis of organs in B-hTSLP/hTSLPR mice plus. The main organs of B-hTSLP/hTSLPR mice plus were isolated at 12 weeks of age and analyzed with H&E staining (male, n=10; female, n=10). Results showed that no obvious abnormalities were found in all of the organs (brain, heart, lung, liver, spleen, stomach, small intestine, colon, kidney, ovary, uterus ,testis, lymph node, thymus and bone marrow).
Q1: What are TSLP and TSLPR?
TSLP is an epithelial-derived cytokine, and TSLPR (CRLF2) is its receptor on immune cells. Together, they regulate inflammation and allergy responses.
Q2: Why use B-hTSLP/hTSLPR mice plus?
They uniquely co-express human TSLP and TSLPR, making them ideal for antibody validation and inflammatory disease modeling.
Q3: What diseases can be studied with this model?
Autoimmune and allergic diseases, including asthma, IBD, and atopic dermatitis.
Q4: Are these mice suitable for antibody validation?
Yes, they are widely used for in vivo validation of therapeutic antibodies targeting the TSLP–TSLPR pathway.
Q5: How do they compare with competitor models?
Unlike single humanized models, B-hTSLP/hTSLPR humanized mice offer dual-target humanization, providing higher translational relevance.