
NOD.CB17-Prkdcscid Il2rgtm1Bcgen H2-K1tm1Bcgen H2-Ab1tm1Bcgen H2-D1tm1Bcgen/Bcgen • 112946

| Product name | B-NDG MHC I/II DKO mice ad |
|---|---|
| Catalog number | 112946 |
| Strain name | NOD.CB17-Prkdcscid Il2rgtm1Bcgen H2-K1tm1Bcgen H2-Ab1tm1Bcgen H2-D1tm1Bcgen/Bcgen |
| Strain background | B-NDG |
| NCBI gene ID | 14972,14964,14961,16186,19090 (Mouse) |
| Aliases | K-f; H-2K; H2-K; H2-D1; H-2K(d); H-2D; H2-D; H2-K1; IAb; Ia2; Ia-2; Abeta; H-2Ab; H2-Ab; Rmcs1; I-Abeta; gc; p64; [g]c; CD132; gamma(c); p460; scid; slip; DNAPK; DNPK1; HYRC1; XRCC7; dxnph; DOXNPH; DNAPDcs; DNA-PKcs |
Gene targeting strategy for B-NDG MHC I/II DKO mice ad. The exons 2-8 of mouse H2-K1 and H2-D1 gene, the exons 2-4 of mouse H2-Ab1 gene were knocked out in B-NDG MHC I/II DKO mice ad. So the transcription and translation of mouse H2-K1, H2-D1, H2-Ab1 genes will be disrupted.
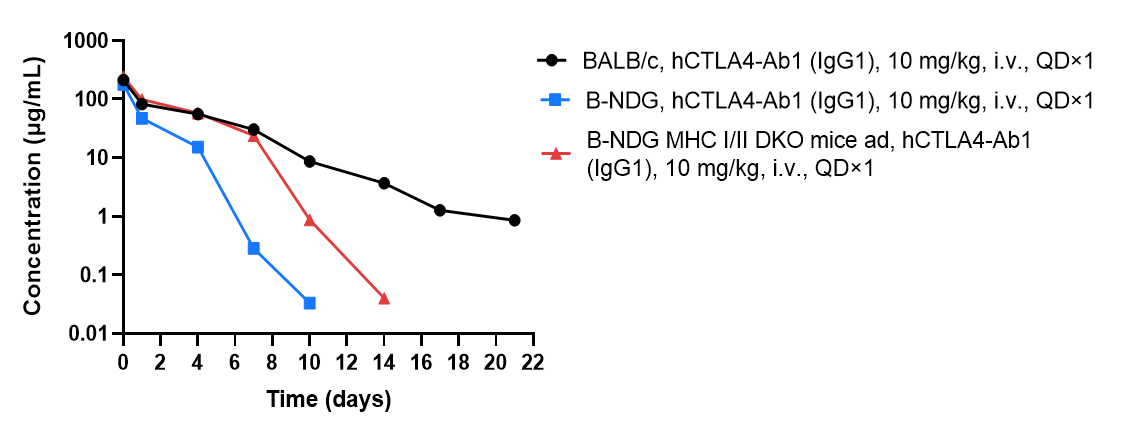
The elimination of human IgG1 antibody in the plasma of B-NDG MHC I/II DKO mice ad was significantly slower than that in B-NDG mice. BALB/c mice (n=5), B-NDG mice (n=4) B-NDG MHC I/II DKO mice ad (n=4) were treated with anti-human CTLA4 antibody (hCTLA4-Ab1). Blood samples were collected at different time point for the PK assay. Results showed that B-NDG MHC I/II DKO mice ad can significantly extend the half-life of antibody drugs in mice.
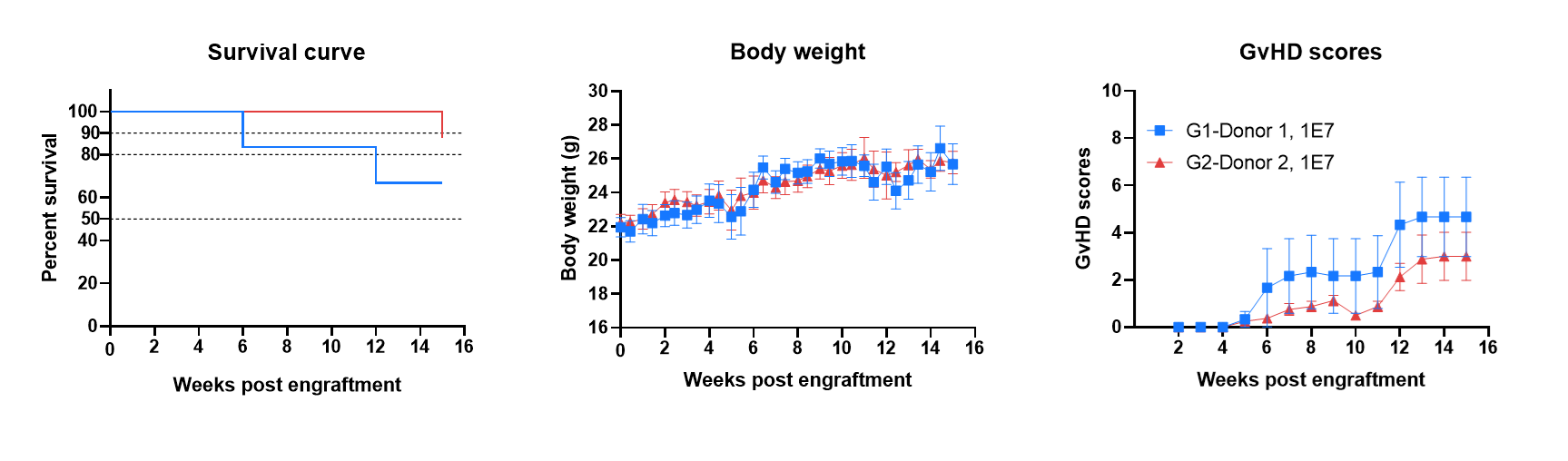
B-NDG MHC I/II DKO mice ad has a long life span and reduced severity of GvHD when engrafted with human PBMCs. B-NDG MHC I/II DKO mice ad were engrafted intravenously with human PBMCs (1×107) from two donors (Donor 1, Donor 2) on day 0 (n=6/8). Survival rates of the mice were analyzed with Kaplan Meier survival curves. Body weight was measured twice a week. Clinical signs of GvHD were scored once a week. Euthanasia was implemented when the body weight decreased more than 20%. Meanwhile, the GvHD score of the mouse was recorded as 10. Values were expressed as mean ± SEM.
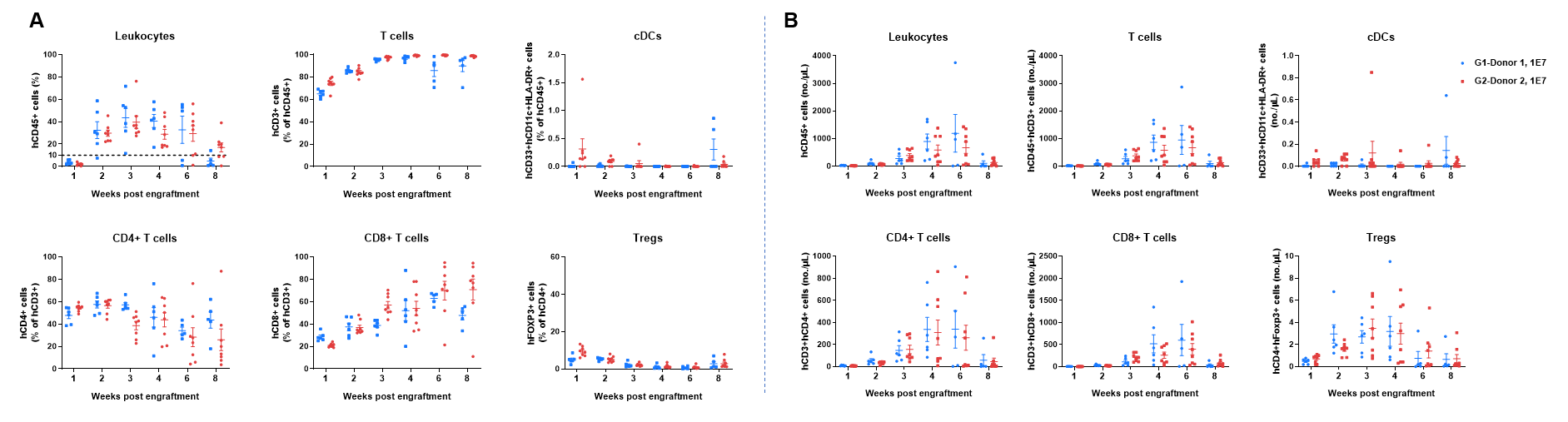
Human PBMCs were successfully reconstituted in B-NDG MHC I/II DKO mice ad. B-NDG MHC I/II DKO mice ad were engrafted intravenously with human PBMCs (1×107) from two donors on day 0 (n=6/8). Peripheral blood was taken to analyze the reconstitution level of human immune cells. A. Frequency of reconstituted human immune cells; B. Absolute cell number of reconstituted human immune cells. The results showed that two weeks after the reconstitution of human PBMCs in B-NDG MHC I/II DKO mice ad, the frequency and absolute cell number of CD45+ cells in peripheral blood began to increase. The frequency of reconstituted human T cells exceeded 90% from three weeks and continued to rise, eventually reaching nearly 100%. Reconstituted human T cells include CD4+T cells, CD8+T cells and Tregs. A small amount of DCs can also be detected. This indicates that B-NDG MHC I/II DKO mice ad is a powerful immunodeficient mouse model for reconstitution of the human T cells using human PBMCs.
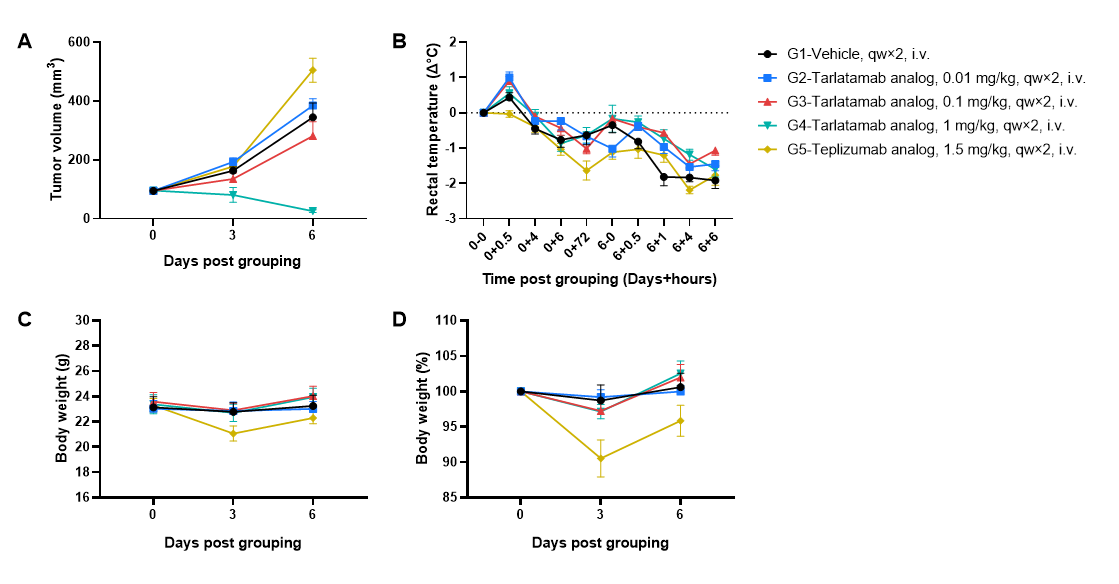
Antitumor activity and toxicity of anti-human CD3/DLL3 bispecific T-cell engager-tarlatamab analog (in-house) in small cell lung cancer cell line SHP-77 CDX model established with huPBMC-B-NDG MHC I/II DKO mice ad . Human PBMCs (1×107) were intravenously engrafted into B-NDG MHC I/II DKO mice ad. Seven days later, SHP-77 cells (5×106) were subcutaneously inoculated into huPBMC-B-NDG MHC I/II DKO mice ad. Mice were grouped when the tumor volume reached approximately 100 mm3, and the frequency of hCD45+ cells ≥10%, at which time they were treated intravenously with tarlatamab analog and teplizumab analog as positive control for CRS (Cytokine Release Syndrome). The doses and schedule were indicated in panel (female, 8-week-old, n=6). (A) Tarlatamab analog could effectively inhibit tumor growth in a dose-dependent manner in huPBMC-B-NDG MHC I/II DKO mice ad, whereas teplizumab analog showed no tumor antigen–dependent antitumor activity. (B–D) Teplizumab analog induced pronounced body weight loss and hypothermia, while tarlatamab analog caused only mild body weight changes with measurable but less severe decreases in rectal temperature. Values are expressed as mean ± SEM.
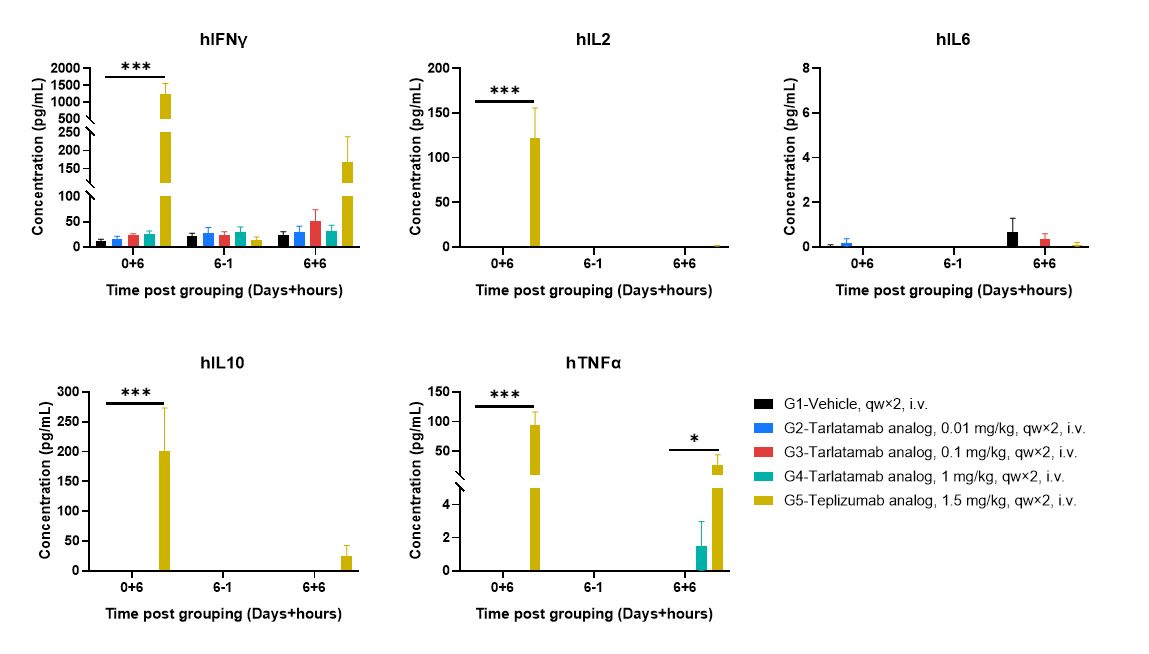
Plasma cytokine releases following first and repeat dosing in huPBMC-B-NDG MHC I/II DKO mice ad. Plasma cytokine levels were assessed at defined time points following dosing, including 6 hours after the first administration (0+6), one day prior to the second administration on day 6 (6−1), and 6 hours after the second administration (6+6). Cytokine concentrations were higher following the first administration than after the second administration, indicating a more pronounced cytokine response after initial dosing. Teplizumab analog-treated mice exhibited elevated cytokine levels, whereas cytokine levels in tarlatamab analog-treated groups were low or below the detection range at the indicated time points. The undetectable values may be attributable to high plasma dilution during sample preparation, and assay sensitivity will be further optimized by reducing dilution factors in subsequent studies. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001.
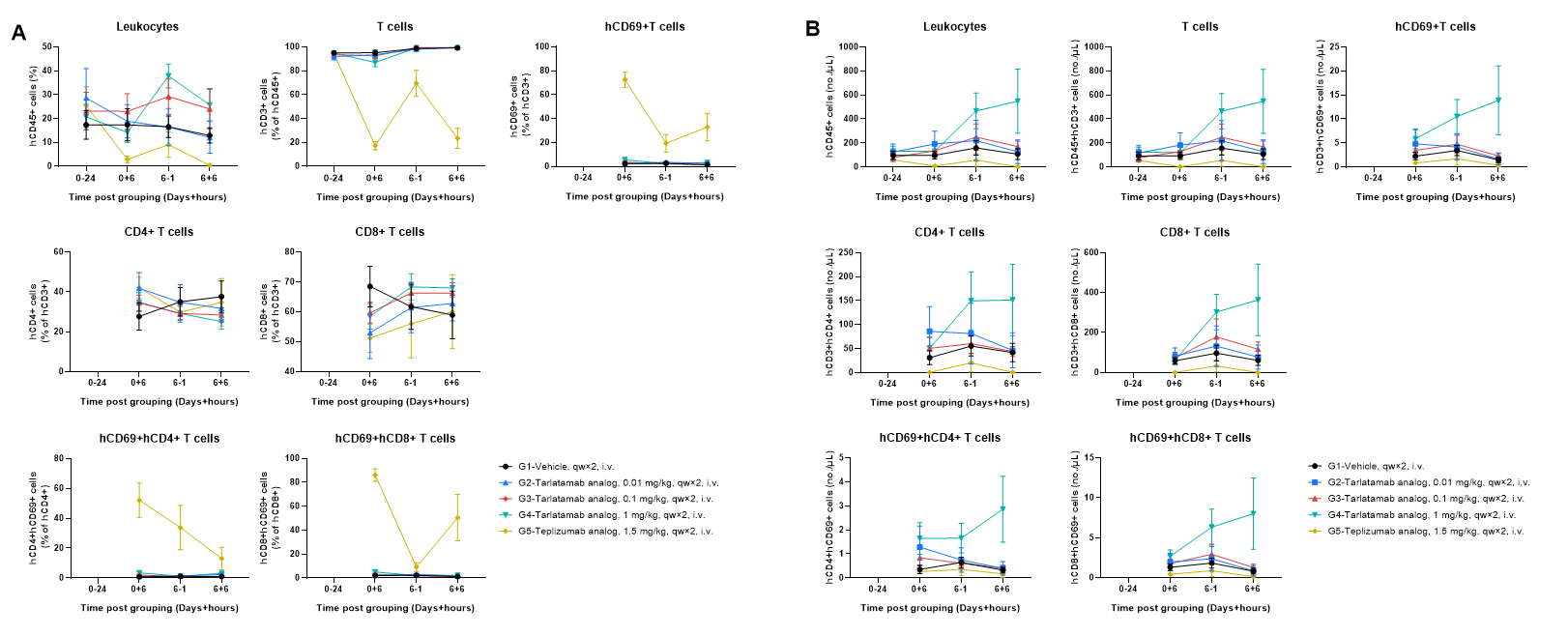
Human PBMCs were successfully reconstituted in B-NDG MHC I/II DKO mice ad. Peripheral blood was collected at the indicated time points. (A) The frequencies of reconstituted immune cells; (B) The absolute number of reconstituted immune cells. In vehicle- and tarlatamab analog–treated groups, the frequencies of CD3+ T cells remained high with relatively stable CD4/CD8 composition, while the 1 mg/kg tarlatamab analog group exhibited a clear increase in circulating human CD45+ leukocytes and CD3+ T cell counts after dosing, accompanied by a modest increase in CD69+ T cells. In contrast, the teplizumab analog (positive control for CRS) induced rapid and pronounced CD69 upregulation on both CD4+ and CD8+ T cells, together with an overall reduction in circulating human leukocyte/T cell frequency and absolute numbers compared with other groups. Values are expressed as mean ± SEM.
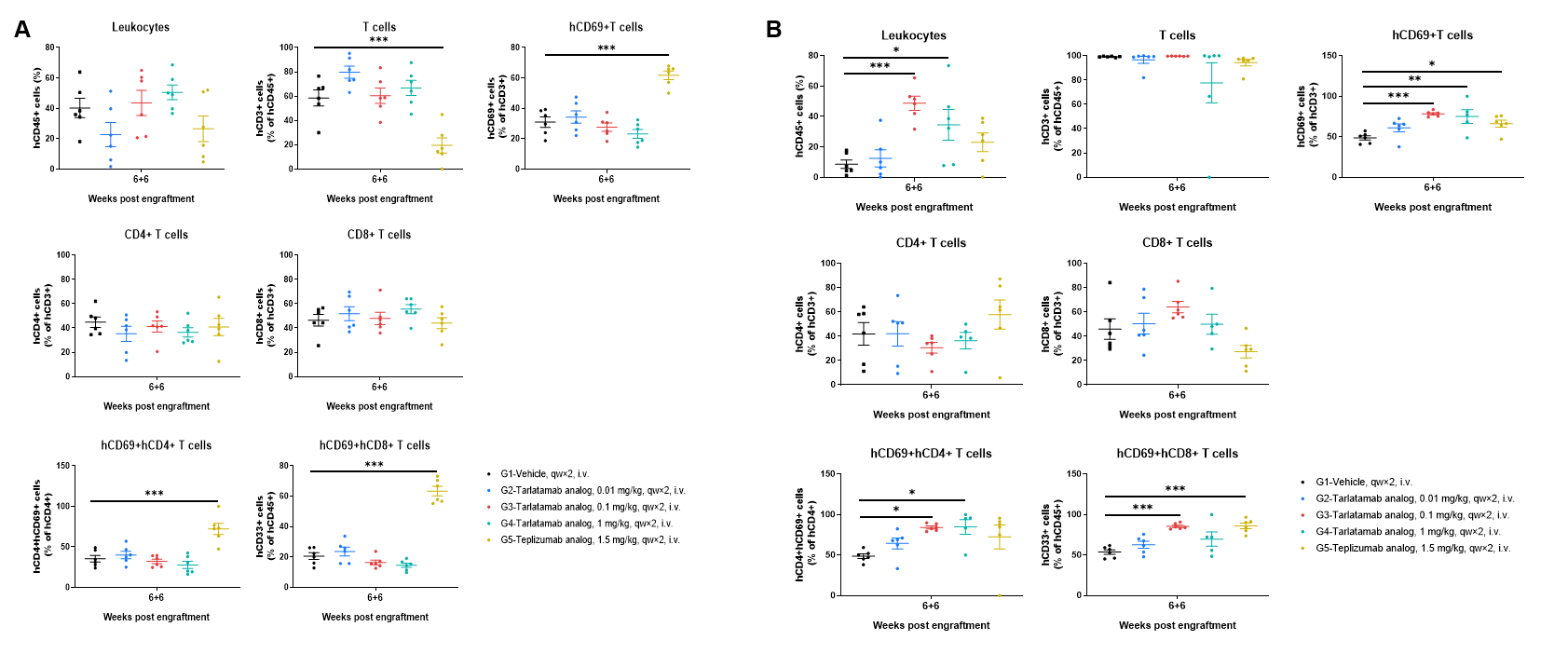
The frequencies of human immune cells in spleen and tumor at 6 h after the second administration. Spleen (A) and tumor tissues (B) were harvested and immune cells were analyzed by flow cytometry. Across the vehicle and tarlatamab analog–treated groups, the overall frequencies of human CD45+ leukocytes and human CD3+ T cells in both spleen and tumor were generally comparable, and the CD4+/CD8+ composition within the T-cell compartment showed no marked shift. In contrast, the teplizumab analog (CRS positive control) induced a pronounced increase in T-cell activation, as evidenced by elevated frequencies of CD69+ T cells as well as CD69+CD4+ and CD69+CD8+ subsets in both spleen and tumor, accompanied by a lower frequency of CD3+ T cells among hCD45+ cells compared with the other groups. Values are expressed as mean ± SEM. Significance was determined by one-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001.
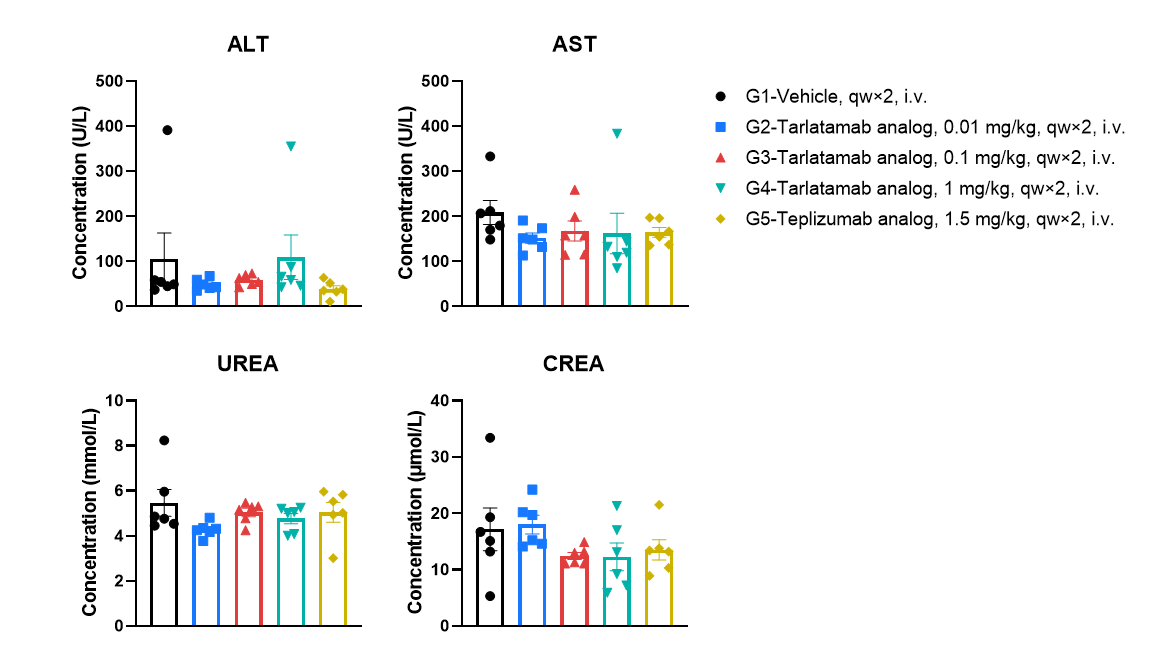
No apparent acute hepatic or renal toxicity signals at 6 h after the second administration. Serum was collected 6 h after the second intravenous administration and clinical chemistry parameters were measured, including ALT and AST (liver enzymes) as well as urea and creatinine (renal function markers). Values are expressed as mean ± SEM. Significance was determined by one-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001.