Description
- PD-1 (CD279) is an inhibitory immune checkpoint receptor composed of an extracellular IgV-like domain, a transmembrane region, and a cytoplasmic tail containing immunoreceptor tyrosine-based inhibitory (ITIM) and switch (ITSM) motifs. Its principal ligands, PD-L1 (CD274) and PD-L2 (CD273), are expressed on antigen-presenting cells, tumor cells, and various stromal cells. Upon ligand engagement, PD-1 recruits SHP-2 phosphatase, leading to dephosphorylation of key signaling molecules downstream of the T-cell receptor (TCR) and CD28 pathways. This results in attenuation of T-cell proliferation, cytokine production, and effector function, thereby maintaining peripheral tolerance but also enabling tumor immune evasion.
- CD28 is a canonical co-stimulatory receptor required for full T-cell activation. Structurally, CD28 consists of an extracellular IgV-like domain, a transmembrane region, and a cytoplasmic tail containing signaling motifs that recruit PI3K, Grb2, and other adaptor proteins. CD28 primarily binds to B7 family ligands CD80 (B7-1) and CD86 (B7-2) expressed on antigen-presenting cells. Engagement of CD28 synergizes with TCR signaling to promote T-cell activation, survival, metabolic reprogramming, and interleukin-2 (IL-2) production. In the absence of CD28 co-stimulation, TCR engagement may result in T-cell anergy or deletion.
- Importantly, PD-1 exerts its inhibitory function in part by directly suppressing CD28-mediated signaling, highlighting the functional interdependence of these two pathways. This mechanistic link has significant implications for cancer immunotherapy.
- A chimeric CDS that encodes human PD-1 signal peptide and extracellular domain, mouse PD-1 transmembrane and cytoplasmic domain was inserted right after mouse PD-1 ATG to replace the mouse PD-1 gene. The exons 2-3 of mouse CD28 gene that encode the extracellular domain were replaced by human counterparts in B-hCD28 mice. The genomic region of mouse CD28 gene that encodes cytoplasmic portion was retained. The promoter, signal peptide, 5’UTR and 3’UTR region of the mouse gene were also retained.
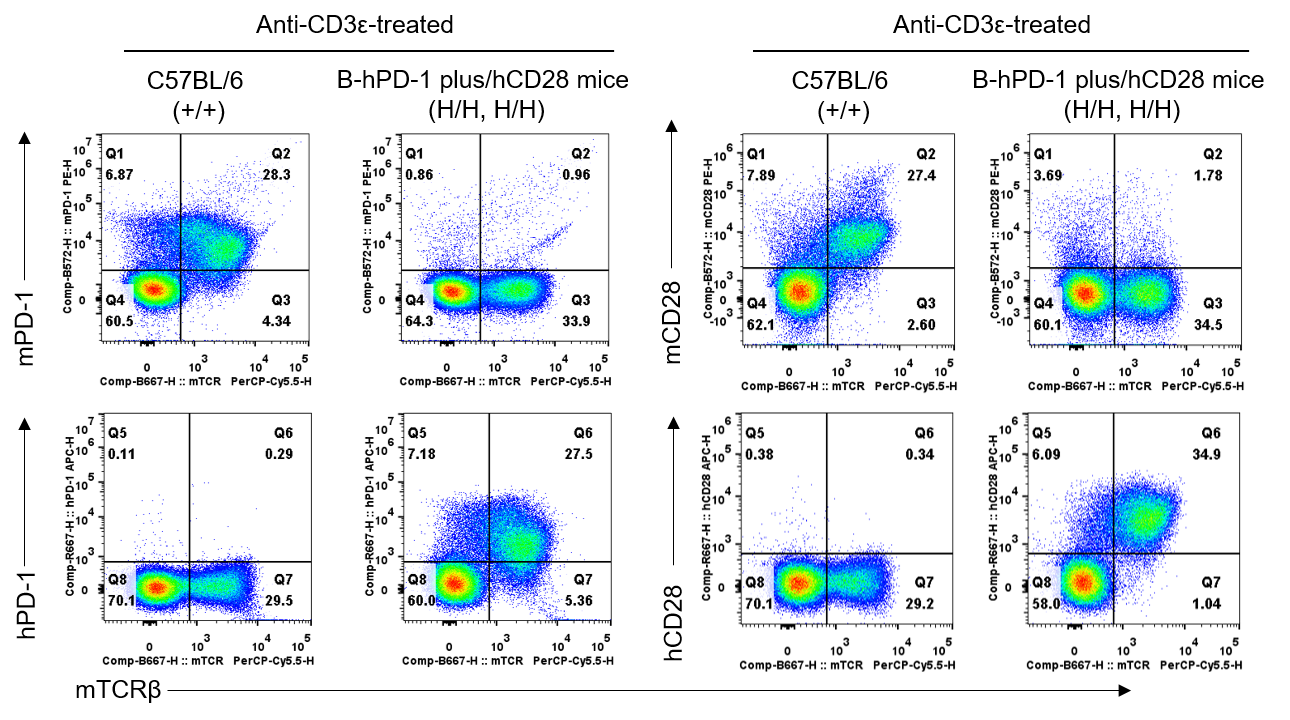
- Mouse PD-1 and CD28 were only detectable in wild-type C57BL/6 mice. Human PD-1 and CD28 were exclusively detectable in homozygous B-hPD-1 plus/hCD28 mice.
- Application: This mouse model empowers the in vivo efficacy and safety evaluation of PD-1/CD28-targeting therapeutic agents.
Targeting Strategy
Gene targeting strategy for B-hPD-1 plus/hCD28 mice.
A chimeric CDS that encodes human PD-1 signal peptide and extracellular domain, mouse PD-1 transmembrane and cytoplasmic domain was inserted right after mouse PD-1 ATG to replace the mouse PD-1 gene. The chimeric PD-1 protein expression will be driven by endogenous mouse PD-1 promoter, while mouse PD-1 gene transcription and translation will be disrupted.
The exons 2-3 of mouse CD28 gene that encode the extracellular domain were replaced by human counterparts in B-hCD28 mice. The genomic region of mouse CD28 gene that encodes cytoplasmic portion was retained. The promoter, signal peptide, 5’UTR and 3’UTR region of the mouse gene were also retained. The chimeric CD28 expression was driven by endogenous mouse CD28 promoter, while mouse CD28 gene transcription and translation will be disrupted.
Protein Expression Analysis in Spleen
PD-1 and CD28 expression analysis in wild-type C57BL/6 mice and homozygous humanized B-hPD-1 plus/hCD28 mice by flow cytometry. Splenocytes were collected from C57BL/6 mice (+/+) and B-hPD-1 plus/hCD28 mice (H/H, H/H) stimulated with anti-mouse CD3ε antibody (7.5 μg, i.p.) in vivo for 24 hrs (female, 8-week-old, n=1). Protein expression was analyzed with anti-mouse PD-1 antibody (Biolegend, 109104), anti-human PD-1 antibody (Biolegend, 329904), anti-mouse CD28 antibody (Biolegend, 102106), and anti-human CD28 antibody (Biolegend, 302912) by flow cytometry. Mouse PD-1 and CD28 were only detectable in wild-type C57BL/6 mice. Human PD-1 and CD28 were exclusively detectable in homozygous B-hPD-1 plus/hCD28 mice, but not in wild-type C57BL/6 mice.
Frequency of Leukocyte Subpopulations in Spleen
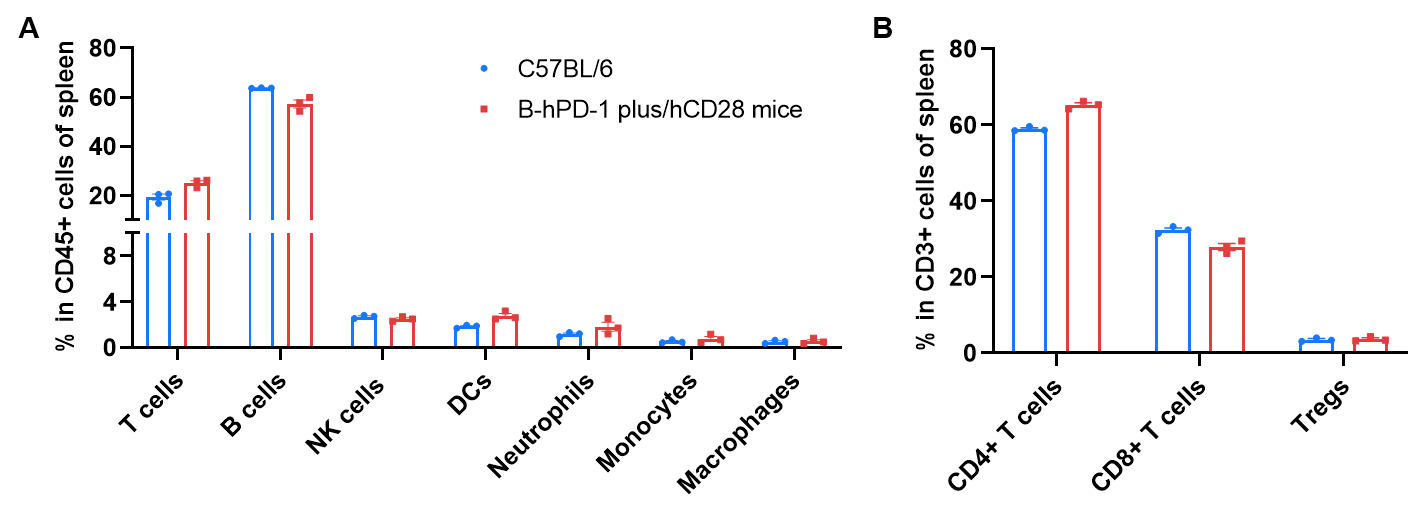
Frequency of leukocyte subpopulations in spleen by flow cytometry. Splenocytes were isolated from wild-type C57BL/6 mice and homozygous B-hPD-1 plus/hCD28 mice (female, 8-week-old, n=3). A. Flow cytometry analysis of the splenocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Frequencies of T cells, B cells, NK cells, DCs, granulocytes, monocytes, macrophages, CD4+ T cells, CD8+ T cells and Tregs in B-hPD-1 plus/hCD28 mice were similar to those in C57BL/6 mice, demonstrating that humanization of PD-1 and CD28 does not change the frequency or distribution of these cell types in spleen. The frequency of leukocyte subpopulations in blood and lymph nodes of B-hPD-1 plus/hCD28 mice were also comparable to wild-type C57BL/6 mice (Data not shown).
* When publishing results obtained using this animal model, please acknowledge the source as follows: The animal model [B-hPD-1 plus/hCD28 mice] (Cat# 113780) was purchased from Biocytogen.