
C57BL/6-Il4tm2(IL4)BcgenIl4ratm1(IL4RA)Bcgen/Bcgen • 120551
Biocytogen's B-hIL4/hIL4RA mice are double humanized IL-4/IL-4Rα mouse models engineered for preclinical research on human cytokine signaling, particularly in allergic inflammation. In these models, exons 1–4 of the mouse Il4 gene and exons 4–7 of the Il4ra gene are replaced with human IL4 and IL4RA counterparts, resulting in exclusive expression of human IL-4 and IL-4Rα proteins.
These mice maintain normal immune cell profiles and enable functional studies of human immune responses. Splenic B cells respond to LPS and human IL-4 stimulation by producing IgE—a response effectively blocked by the IL-4Rα antagonist dupilumab. The B-hIL4/hIL4RA mice are ideal for evaluating therapeutic antibodies targeting the human IL-4/IL-4Rα axis in asthma, atopic dermatitis, and other Th2-mediated diseases.
Gene targeting strategy for B-hIL4/hIL4RA mice.
The exons 1-4 of mouse Il4 gene that encode the full length coding sequence were replaced by human IL4 exons 1-4 in B-hIL4/hIL4RA mice.
The exons 4-7 of mouse Il4ra gene that encode the extracellular region coding sequences were replaced by human IL4RA exons 4-7 in B-hIL4/hIL4RA mice.
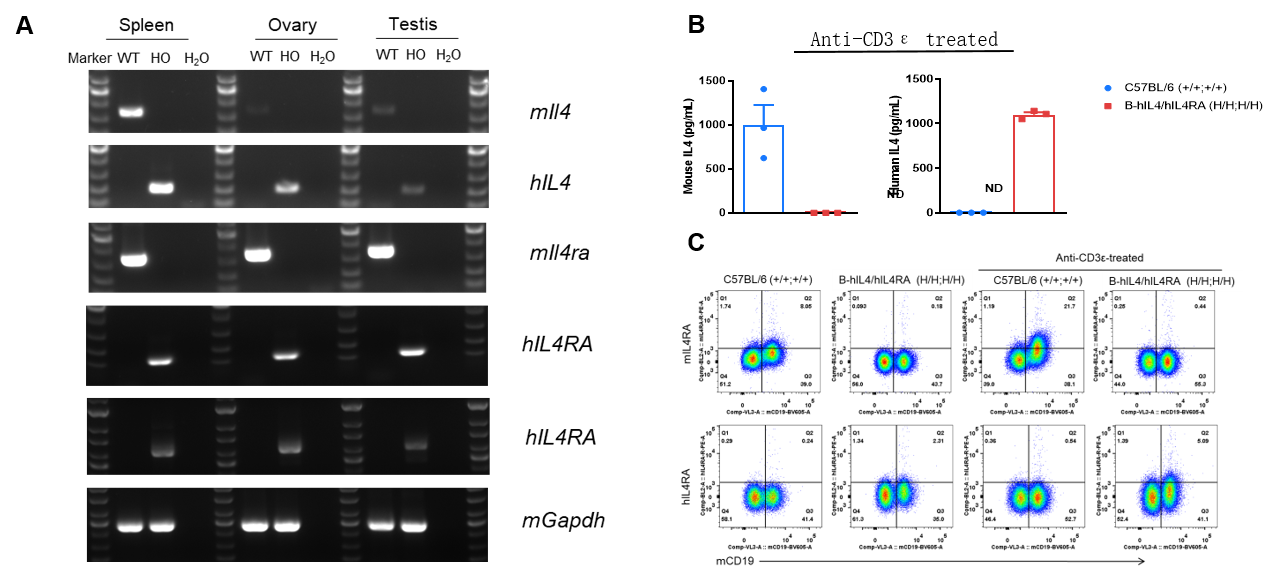
Strain specific analysis of IL4 and IL4RA gene expression in WT and B-hIL4/hIL4RA mice. (A) Mouse Il4 and Il4ra mRNA were detectable in spleen, ovary and testis of wild-type mice. Human IL4 and IL4RA mRNA were detectable only in homozygous B-hIL4/hIL4RA mice but not in wild-type mice. (B) Serum were collected from WT and homozygous B-hIL4/hIL4RA mice stimulated with anti-CD3ε in vivo, and analyzed by ELISA with species-specific IL4 ELISA kit. Mouse Il4 was detectable in WT mice. Human IL4 was exclusively detectable in homozygous B-hIL4/hIL4RA mice but not WT mice. (C) Splenocytes were collected from WT and homozygous B-hIL4/hIL4RA mice, and analyzed by flow cytometry with species-specific anti-IL4RA antibody. Mouse Il4ra was detectable in WT mice. Human IL4RA was exclusively detectable in homozygous B-hIL4/hIL4RA mice but not WT mice.
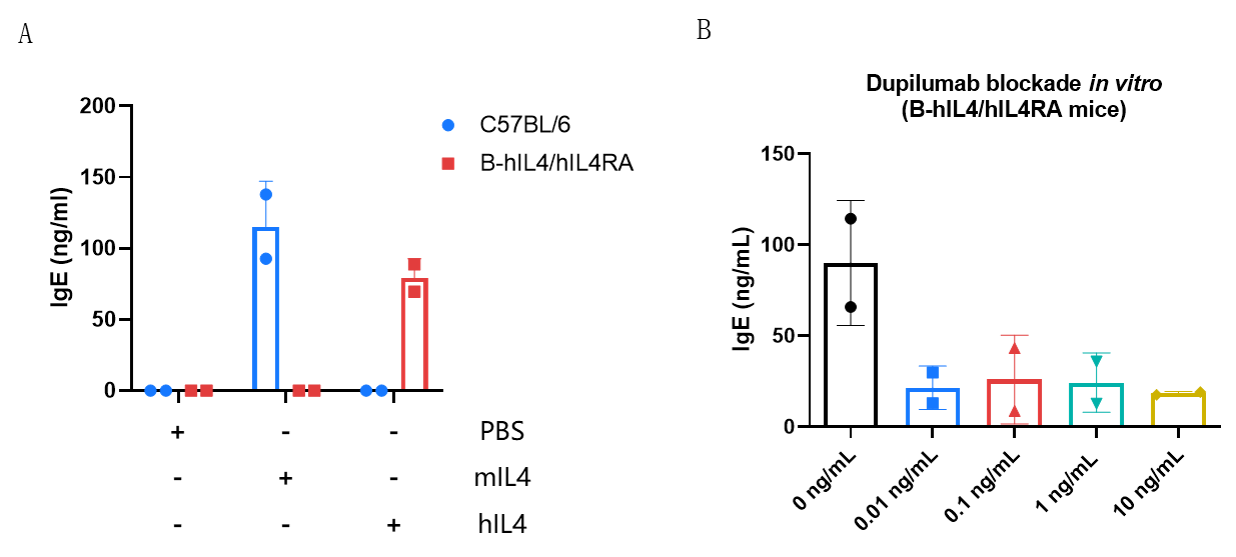
IgE production in B-hIL4/hIL4RA mice and its inhibition by dupilumab (in house). (A) Splenic B cells from C57BL/6 and B-hIL4/hIL4RA mice were cultured with LPS alone or together with 50 ng/mL mIL-4/hIL-4. Culture supplements were harvested on day 6 for IgE quantification by ELISA. (B) Splenic B cells from B-hIL4/hIL4RA mice were incubated with increasing doses of dupilumab (in house) for 30 min before adding LPS and hIL-4 (50 ng/mL). Supernatant was harvested on day 6 for IgE quantification by ELISA. B cells from B-hIL4/hIL4RA mice produced IgE similarly to C57BL/6 mice in response to species-specific IL-4, while dupilumab effectively blocked IgE production.
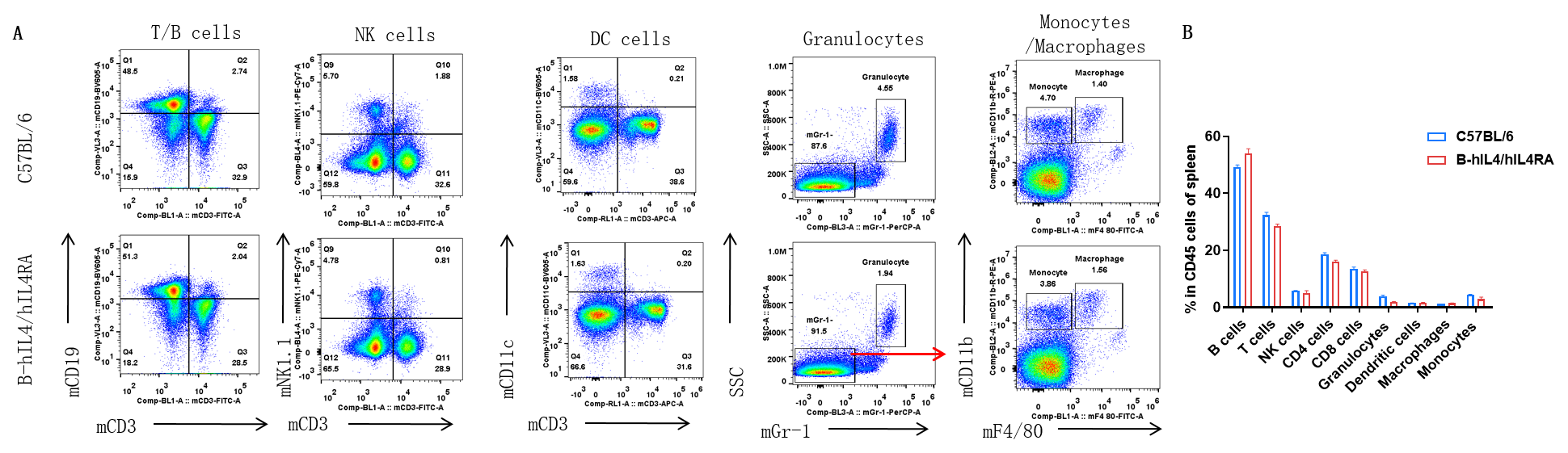
Analysis of spleen leukocyte subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hIL4/hIL4RA mice (n=3, 10-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for CD45 population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T, B, NK, Monocyte, DC and macrophage cells in homozygous B-hIL4/hIL4RA mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL4 and hIL4RA in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in spleen.
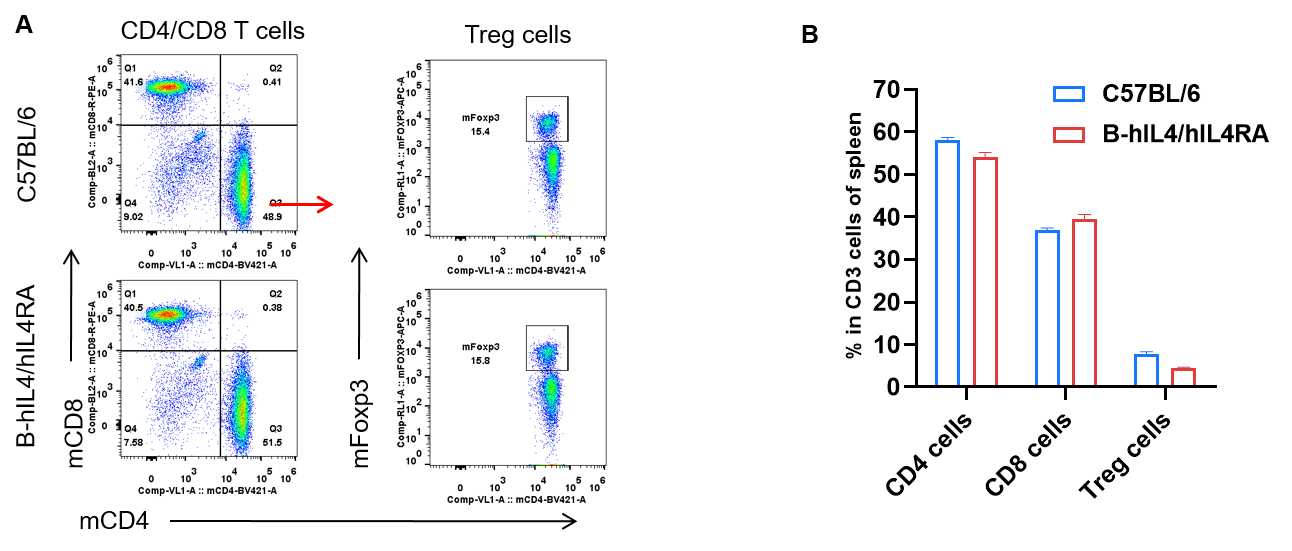
Analysis of spleen T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hIL4/hIL4RA mice (n=3, 10-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ T cells were gated for CD3+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of CD4, CD8, and Treg cells in homozygous B-hIL4/hIL4RA mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL4 and hIL4RA in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell sub types in spleen. Values are expressed as mean ± SEM.
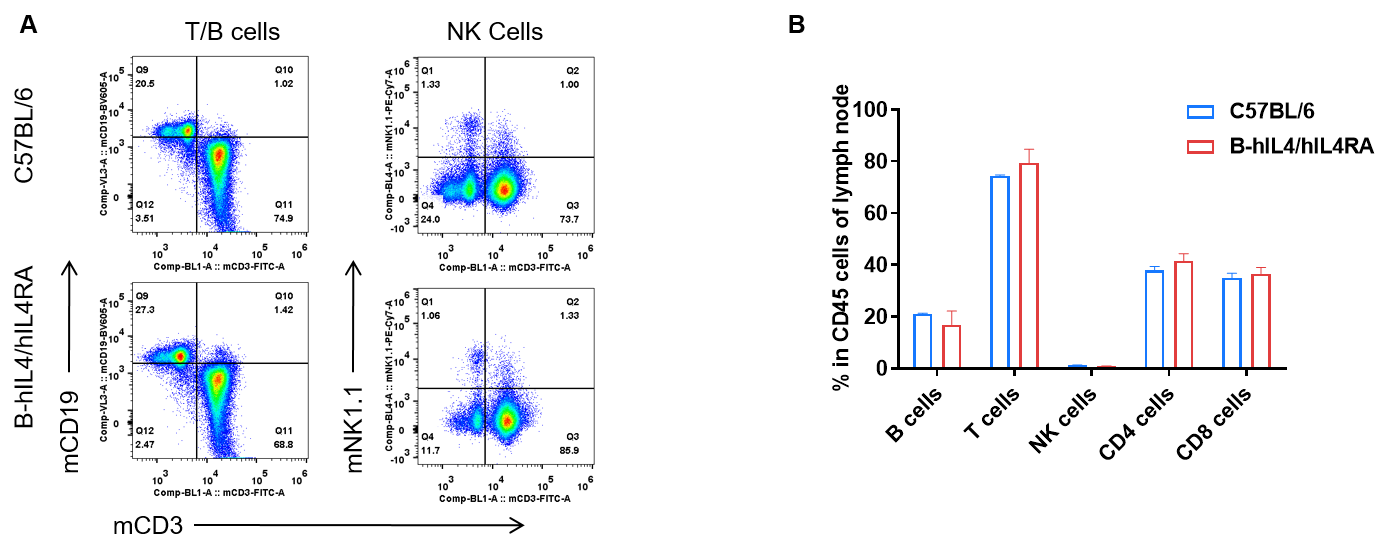
Analysis of subpopulation of leukocytes in lymph node by FACS. Lymph nodes were isolated from female C57BL/6 and B-hIL4/hIL4RA mice (n=3, 10-week-old). Flow cytometry analysis of the lymph nodes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ T cells were used for further analysis as indicated here. B. Results of FACS analysis. Percent of T, B, and NK cells in homozygous B-hIL4/hIL4RA mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL4 and hIL4RA in place of its mouse counterpart does not change the overall development, differentiation or distribution of these cell types in lymph node. Values are expressed as mean ± SEM.
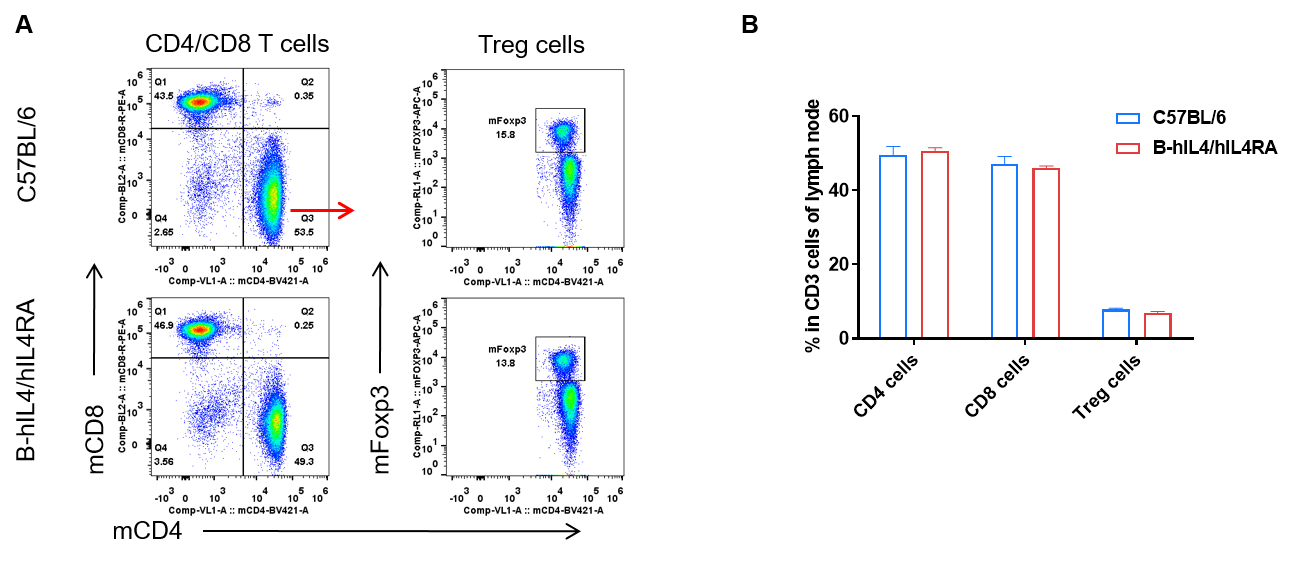
Analysis of subpopulation of T cells in lymph node by FACS. Lymph nodes were isolated from female C57BL/6 and B-hIL4/hIL4RA mice (n=3, 10-week-old). Flow cytometry analysis of the lymph nodes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ T cells were used for further analysis as indicated here. B. Results of FACS analysis. Percent of CD8, CD4, and Treg cells in homozygous B-hIL4/hIL4RA mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hIL4 and hIL4RA in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in lymph node. Values are expressed as mean ± SEM.
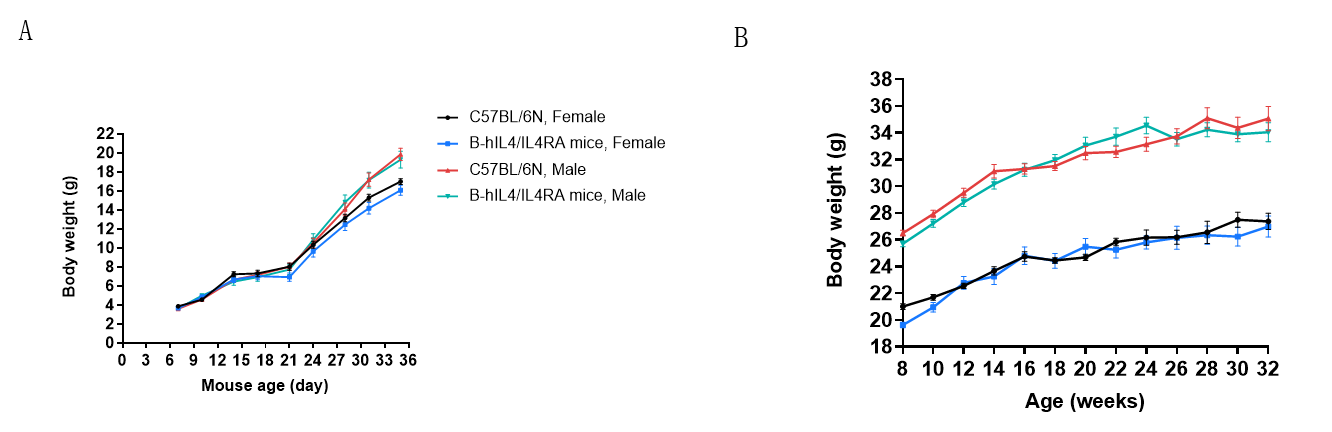
Body weight of wild-type mice and B-hIL4/hIL4RA mice. Wild-type C57BL/6 mice and B-hIL4/hIL4RA mice (10 males and 10 females) were monitored for 1-32 weeks to assess overall health of the animals. (A&B) Absolute body weight and percent weight gain of wild-type mice and B-hIL4/hIL4RA mice over time. The weightB-hIL4/hIL4RA mice revealed no abnormalities compared to wild-type controls.
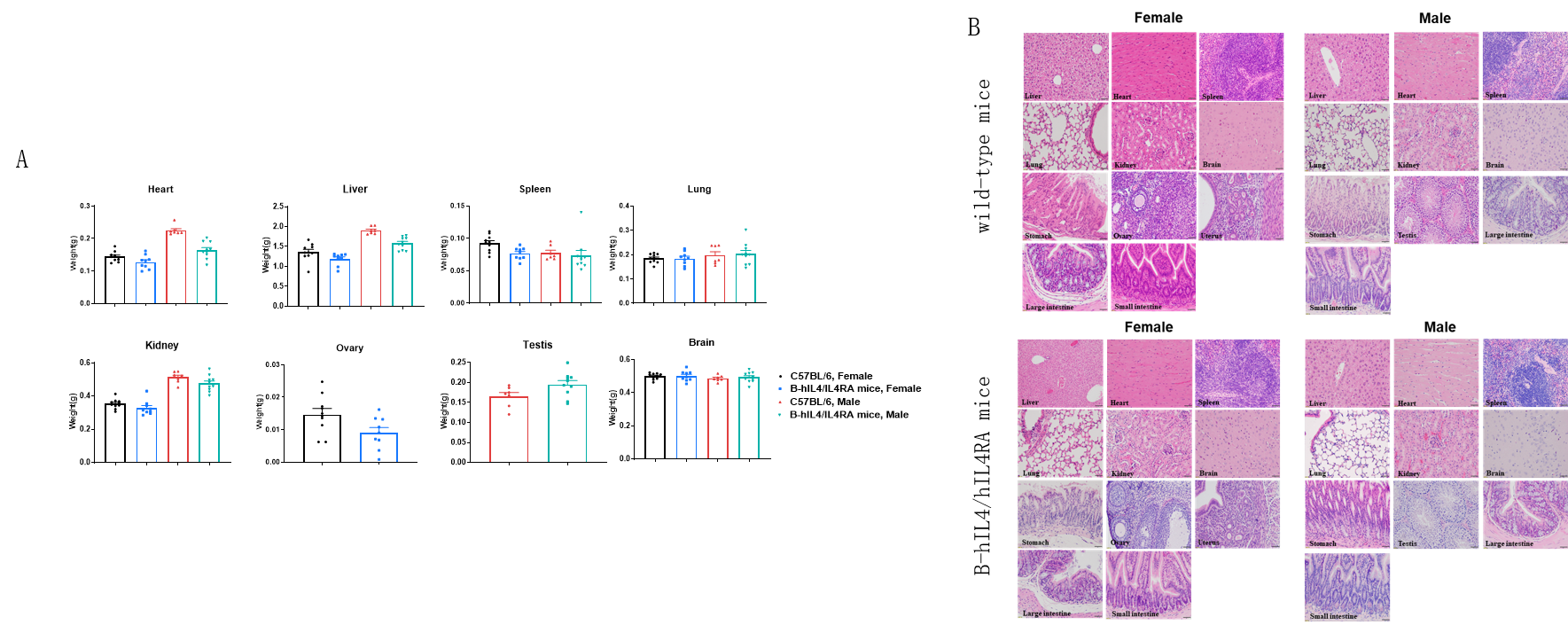
Macroscopic anatomical data of wild-type mice and B-hIL4/hIL4RA mice. 8-week-old wild-type C57BL/6 mice and B-hIL4/hIL4RA mice (10 males and 10 females) were monitored for 32 weeks to assess overall health of the animals. (A) Absolute body weight and percent weight gain of wild-type mice and B-hIL4/hIL4RA mice over time. (A) Organ weight of wild-type mice and B-hIL4/hIL4RA mice.(B) Organ histology (H&E) of wild-type mice and B-hIL4/hIL4RA mice. The morphology of organs and pathological examination of B-hIL4/hIL4RA mice revealed no abnormalities compared to wild-type controls.
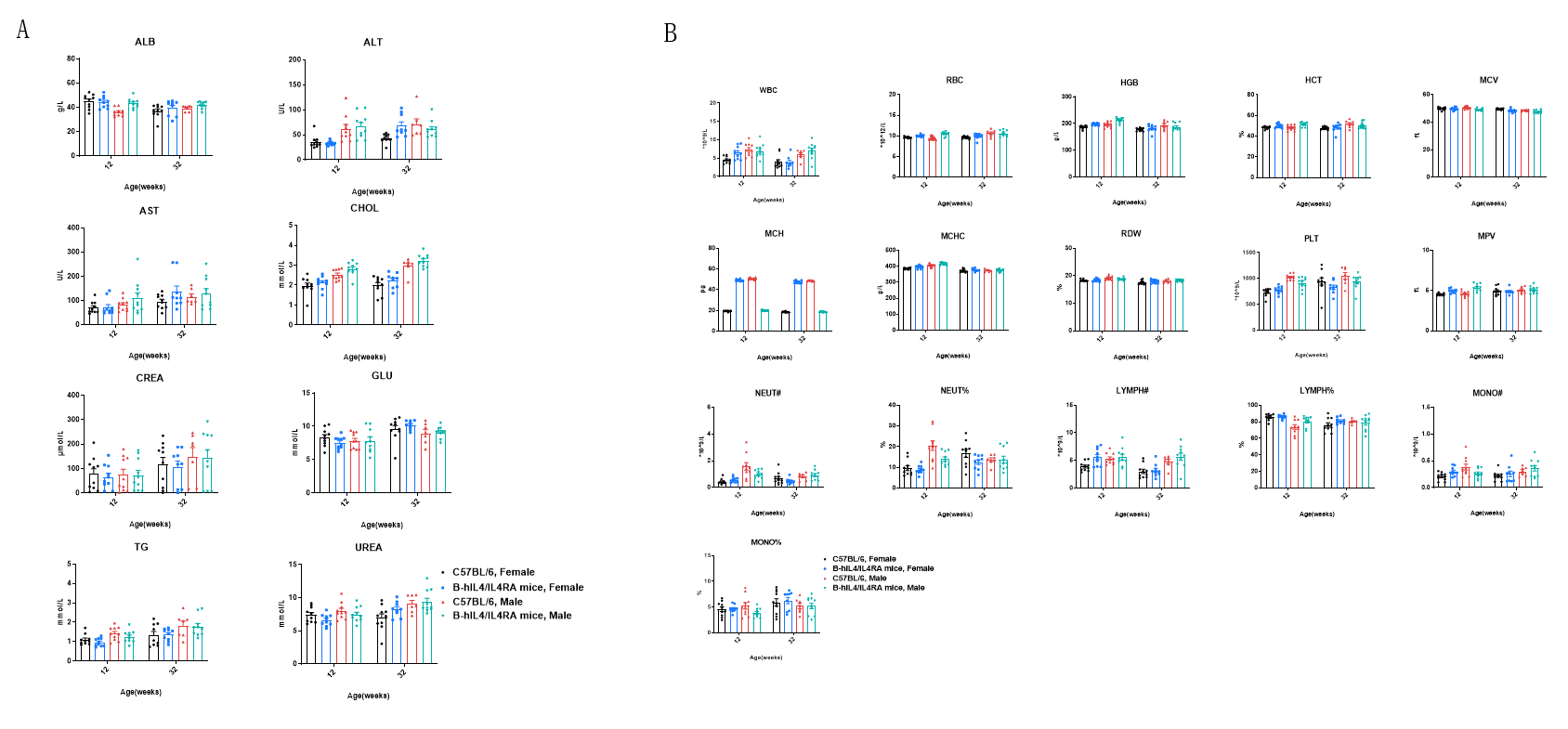
Blood biochemistry and hematology analysis of wild-type mice and B-hIL4/hIL4RA mice measured at 12 and 32 weeks of age. 12-week-old wild-type C57BL/6 mice and B-hIL4/hIL4RA mice (10 males and 10 females) were monitored for 32 weeks to assess overall health of the animals. (A) Biochemistry analysis. (B) Hematology analysis. The data showed that biochemistry analysis and hematology analysis were also similar between B-hIL4/hIL4RA humanized mice and wild-type C57BL/6 mice.
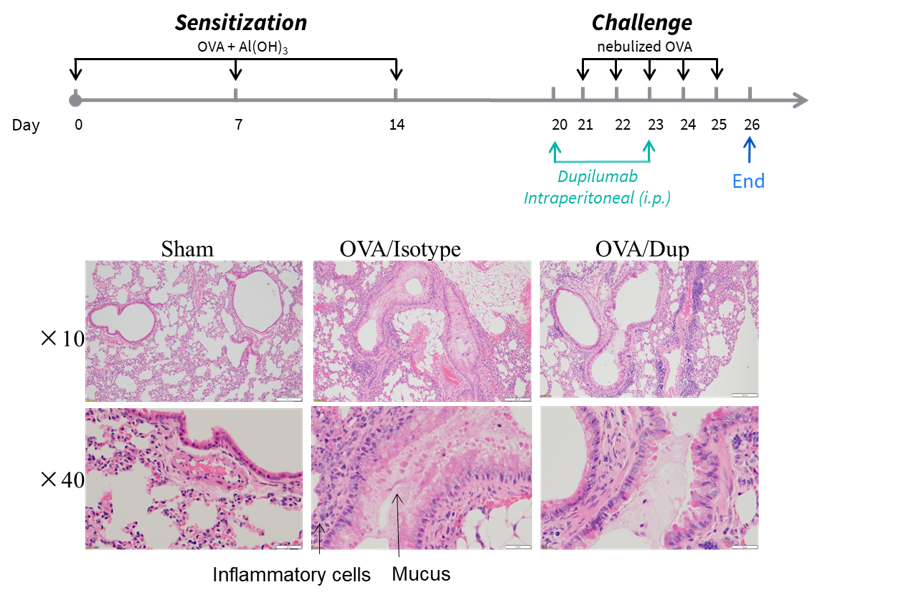
H&E staining of asthma-like model in B-hIL4/hIL4RA mice. Lung tissues were collected at the study endpoint. H&E staining results showed that the lung tissues from B-hIL4/hIL4RA mice exposed to PBS aerosols did not show any inflammation. OVA exposure resulted in a significant increase in peribronchial and perivascular inflammation in B-hIL4/hIL4RA mice. A significant reduction in eosinophils infiltration was observed in mice treated with dupilumab (in house).
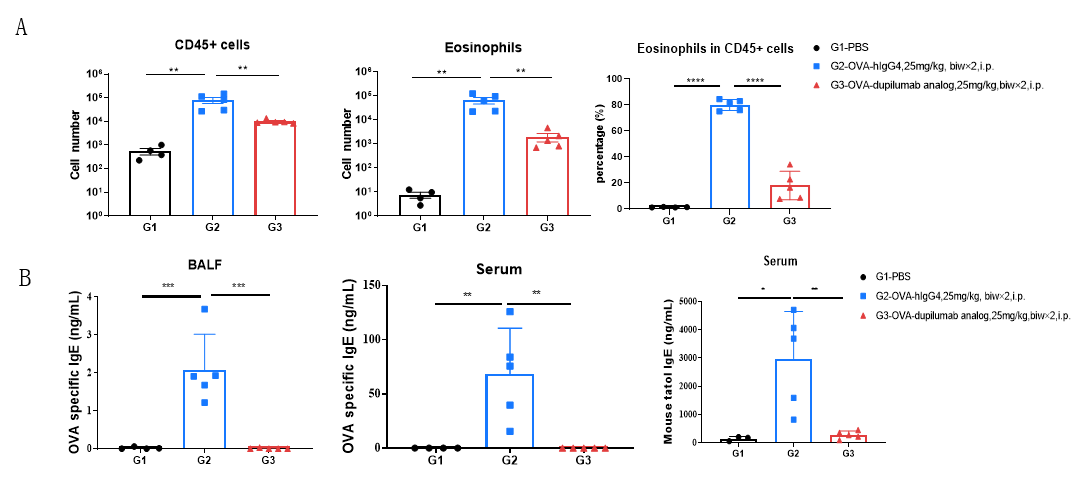
Analysis of immune cells in BALF by FACS and IgE production in serum. BALF immune cells were isolated from B-hIL4/hIL4RA mice (n=4 or n=5). The number and proportion of eosinophils were analyzed by flow cytometry under the treatment of PBS/dupilumab (in house). After treatment of dupilumab (in house), the number of CD45+ cells and eosinophils were much lower than the positive control in homozygous B-hIL4/hIL4RA mice. Serum was collected at the study endpoint. IgE levels responded to OVA-specific antibody and total IgE levels were analyzed. The results show that the levels of IgE in mice treated with dupilumab (in house) is much lower than that in untreated mice.
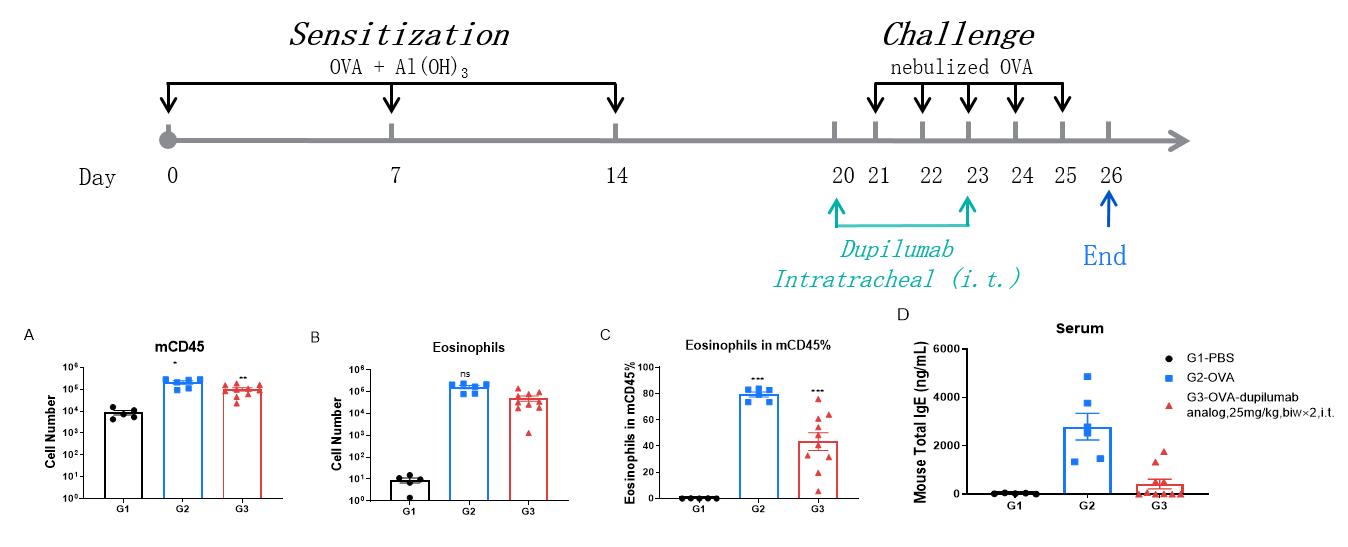
Analysis of immune cells in BALF by FACS and IgE production in serum. BALF immune cells were isolated from B-hIL4/hIL4RA mice. The number and proportion of eosinophils were analyzed by flow cytometry under the treatment of PBS/dupilumab (in house). After treatment of dupilumab (in house), the number of CD45+ cells and eosinophils were much lower than the positive control in homozygous B-hIL4/hIL4RA mice. Serum was collected at the study endpoint. IgE levels were analyzed. The results show that the levels of IgE in mice treated with dupilumab (in house) is much lower than that in untreated mice.
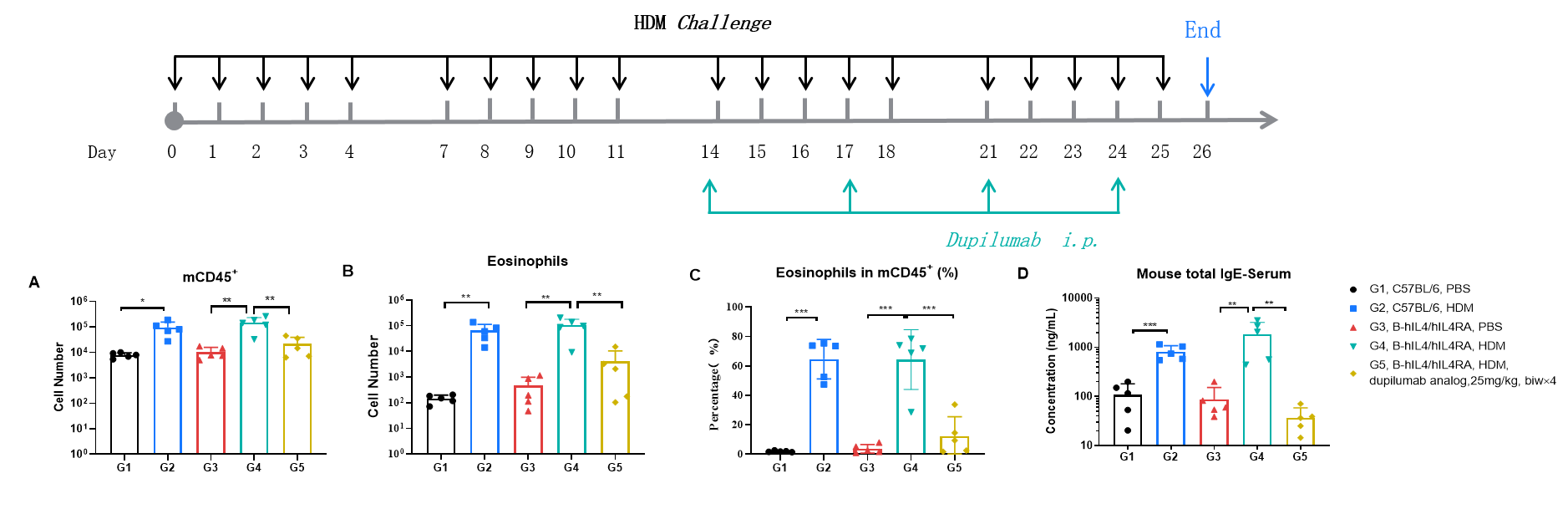
Efficacy Evaluation of the anti-human IL4RA (dupilumab) (in house) in HDM-induced Asthma Model of B-hIL4/hIL4RA mice. (A) The number of CD45+ cells in BALF. (B) The number of eosinophils in BALF. (C) The proportion of eosinophils to CD45+ cells. The results showed that after challenge with HDM, the leukocyte infiltration of mice in G4 model group was significantly increased compared with G3 control group, and their eosinophil content was significantly increased, suggesting that the model was successfully established. After administration of 25 mg/kg dupilumab (in house), the numbers of CD45+ cells and eosinophils were significantly lower compared with the G3 model group. (D) Serum was taken at the end of the experiment and total IgE levels were measured. The results showed that the levels of total IgE in G4 model group were significantly increased compared with G3 control group, suggesting successful modeling. Total IgE levels were significantly lower after administration of dupilumab (in house) drug compared with the G4 model group.
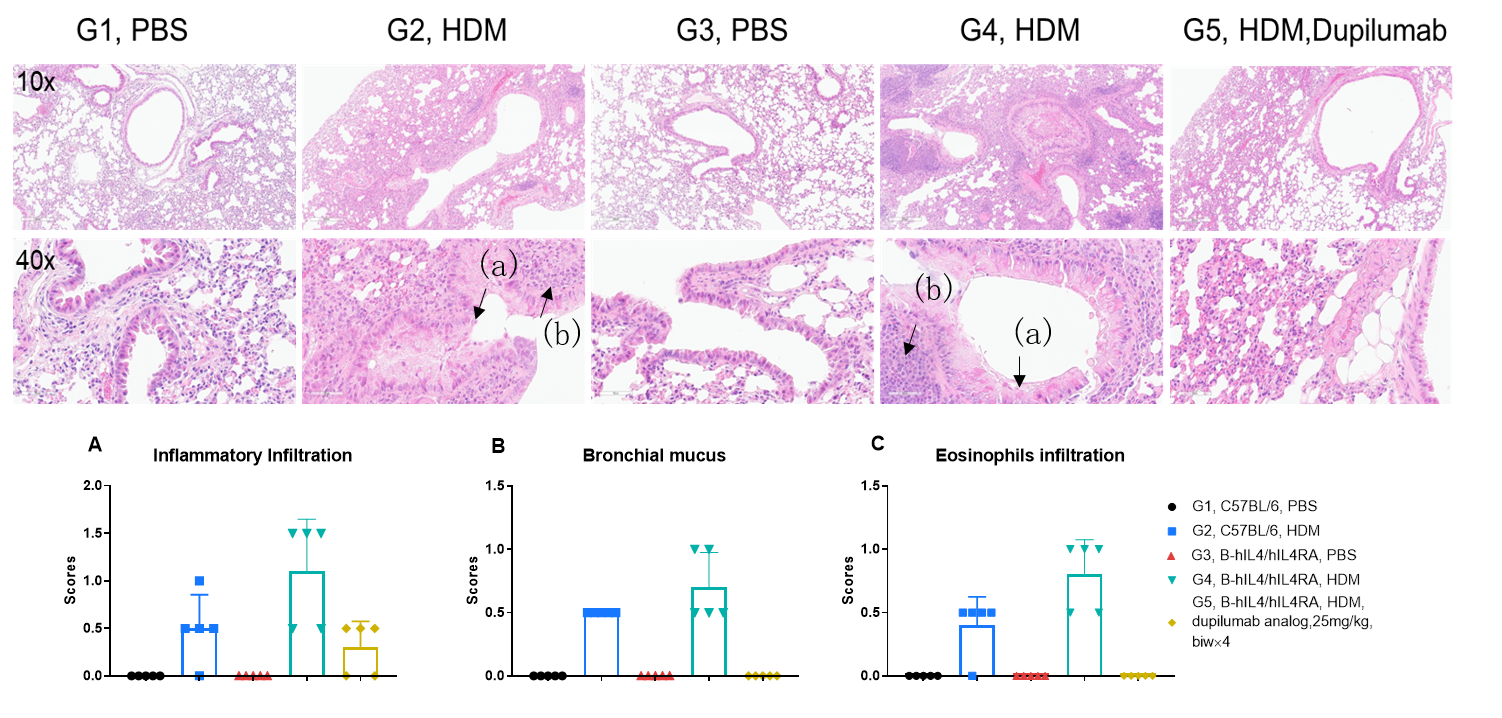
H&E staining in the lungs of asthmatic mice. In contrast to the G1 untreated group, the HDM-treated G2 model group showed asthma-related pathological changes as demonstrated by vascular and peribronchial mixed inflammatory cell infiltration (b) and mucus (a) formation in some bronchi. After administration of dupilumab (in house), the numbers of CD45 + cells and eosinophils were significantly lower compared with the G2 model group.
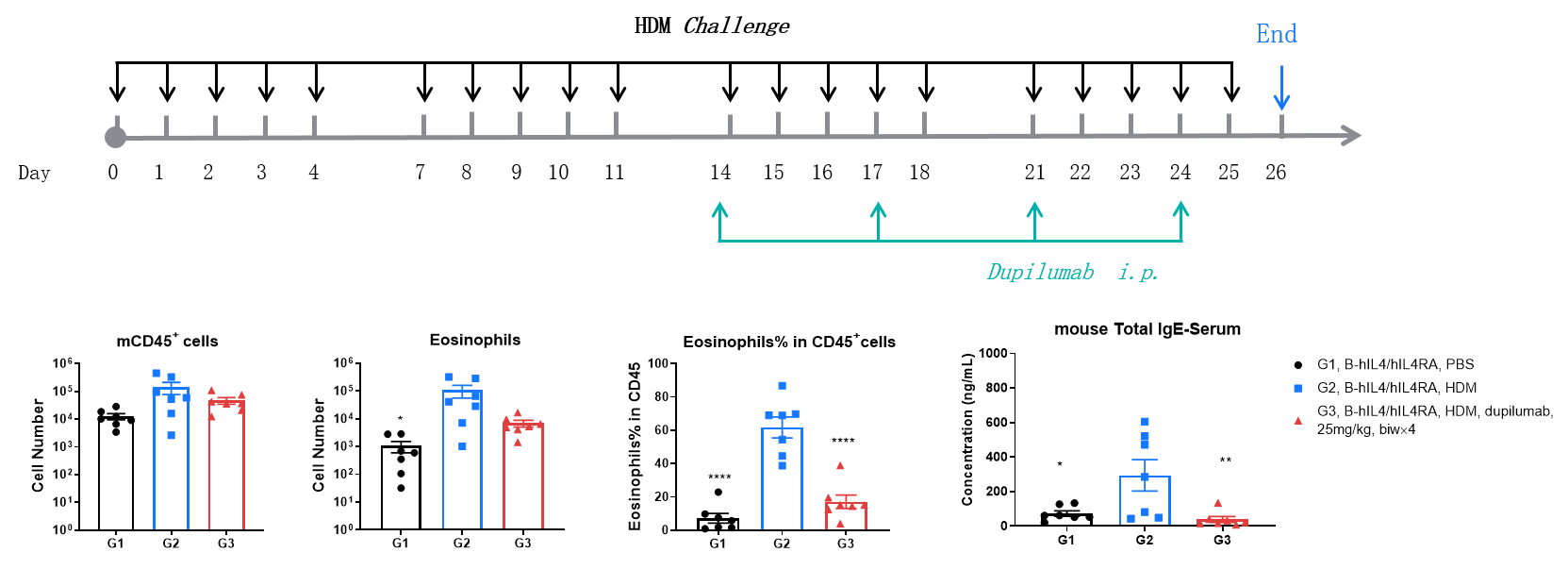
Efficacy Evaluation of the anti-human IL4RA (dupilumab) (in house) in HDM-induced Asthma Model of B-hIL4/hIL4RA mice. ( A) The number of CD45+ cells in BALF. (B) The number of eosinophils in BALF. (C) The proportion of eosinophils to CD45+ cells. The results showed that after sensitization and challenge with HDM, the leukocyte infiltration of mice in G2 model group was significantly increased compared with G1 control group, and their eosinophil content was significantly increased, suggesting that the model was successfully established. After administration of 25 mg/kg dupilumab (in house), the numbers of CD45+ cells and eosinophils were significantly lower compared with the G2 model group. (D) Serum was taken at the end of the experiment and total IgE levels were measured using ELISA. The results showed that the levels of total IgE in G2 model group were significantly increased compared with G1 control group, suggesting successful modeling. Total IgE levels were significantly lower after administration of dupilumab (in house) drug compared with the G2 modeling group. * P < 0.05, ** P < 0.01, **** P < 0.0001 compared with G2 group
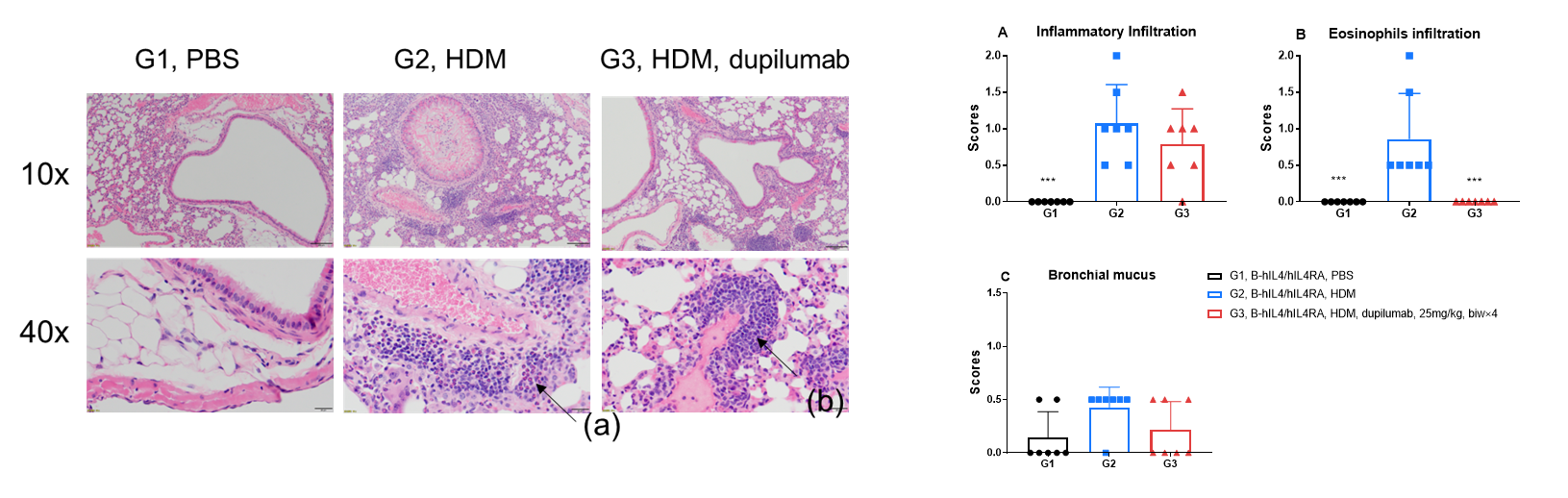
H&E staining in the lungs of asthmatic mice. In contrast to the G1 untreated group, the HDM-treated G2 model animals showed asthma-related pathological changes as demonstrated by vascular and peribronchial mixed eosinophil cell infiltration (a) and inflammatory cell infiltration (b) in some bronchi. After administration of dupilumab (in house), the numbers of mCD45+ cells and eosinophils were significantly lower compared with the G2 model group. *** P < 0.001 compared with G2 group.
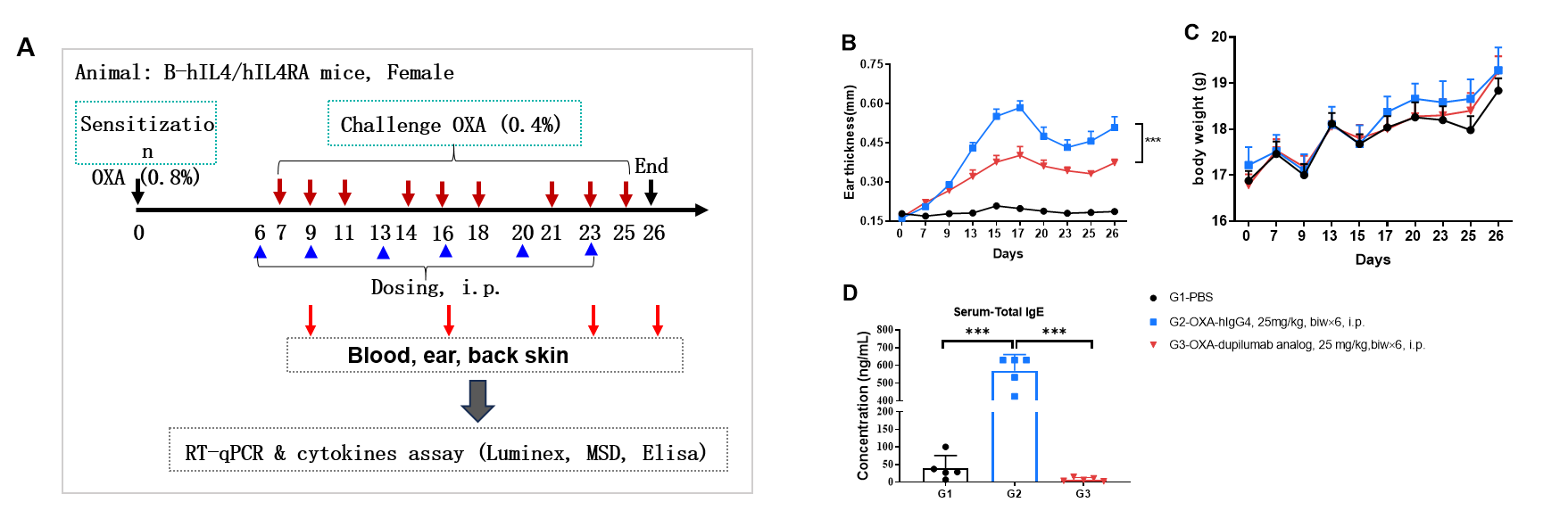
Dupilumab (in house) alleviated OXA induced atopic dermatitis in B-hIL4/IL4RA mice. Mice received 0.8% OXA on ear and skin on day 0, then received 0.4% OXA application three times a week from day 7 to day 25. Body weight and ear thickness were recorded after OXA application. Mice received PBS or dupilumab(in house) (25 mg/kg) twice a week from day 6 for totally 6 times. Ear, back skin and blood were collected at the end (A). Dupilumab produced no effects on body weight (C). Ear thickness (B) and serum IgE (D) were decreased in dupilumab treated mice.
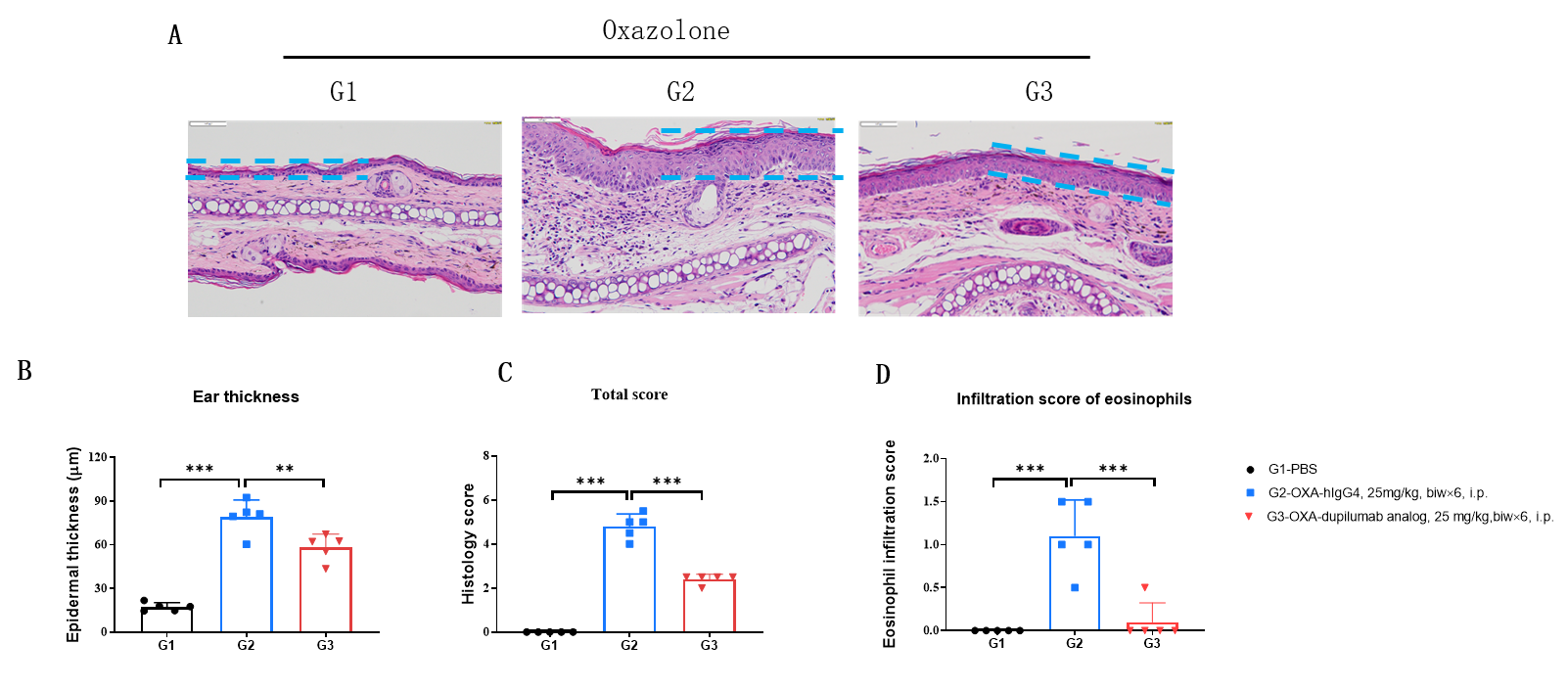
Efficacy of anti-human IL4RA antibody in B-hIL4/hIL4RA mice. Dose dependent effects of dupilumab (in house) in oxazolone induced skin lesions in B-hIL4/hIL4RA mice.
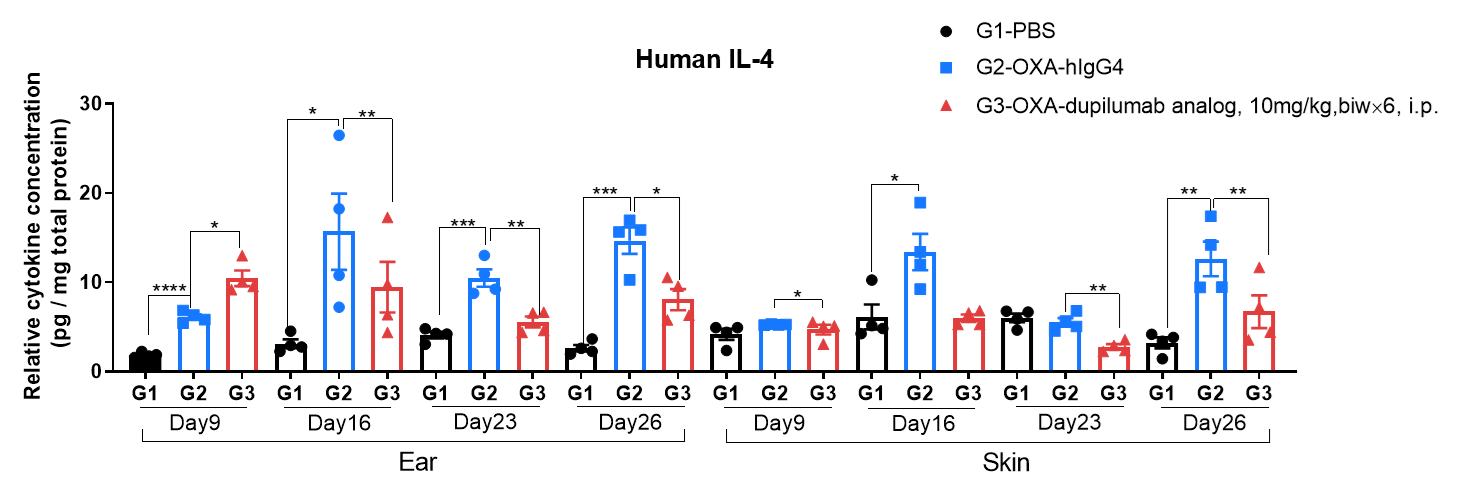
Protein expression analysis in OXA-induced AD model of B-hIL4/hIL4RA mice by ELISA.
Ear and skin samples of modeling area were collected from at day 9, 16, 23 and 26 and analyzed by ELISA. Tissue sample homogenate supernatants were loaded for ELISA detection, and the results were standardized by total protein concentration of the corresponding sample.
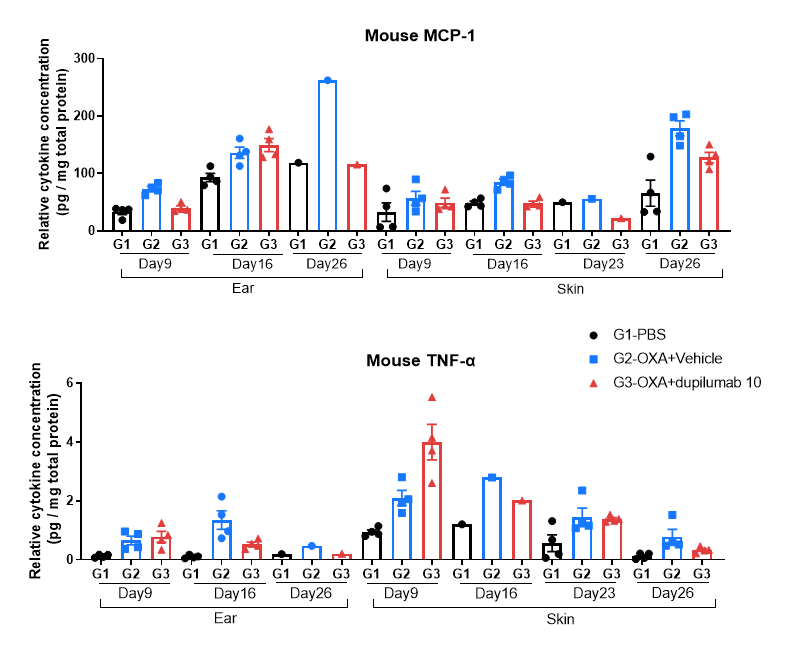
Protein expression analysis in OXA-induced AD model of B-hIL4/hIL4RA mice by Luminex.
Ear and skin samples of modeling area were collected from at day 9, 16, 26 and 26 and analyzed by Luminex. Tissue sample homogenate supernatants were loaded for detection.
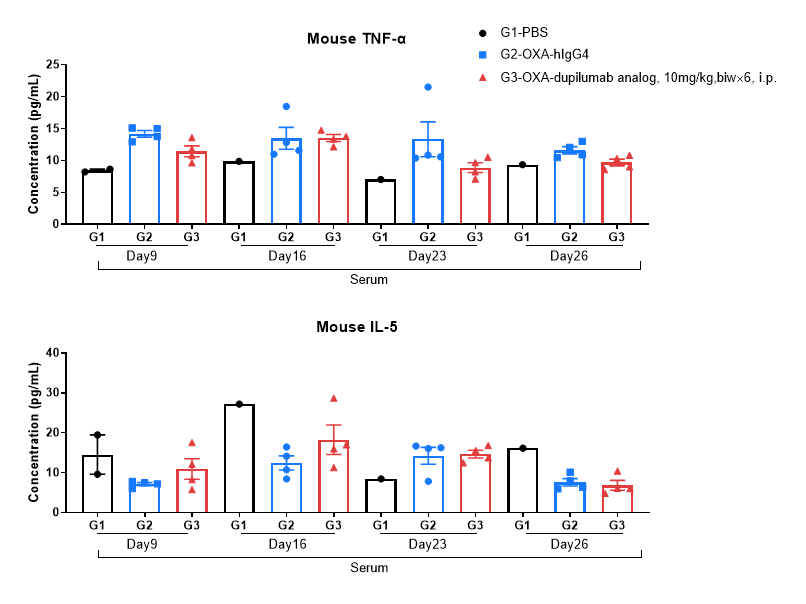
Protein expression analysis in OXA-induced AD model of B-hIL4/hIL4RA mice by MSD.
Ear and skin samples of modeling area were collected from at day 9, 16, 26 and 26 and analyzed by Luminex. Tissue sample homogenate supernatants were loaded for detection.
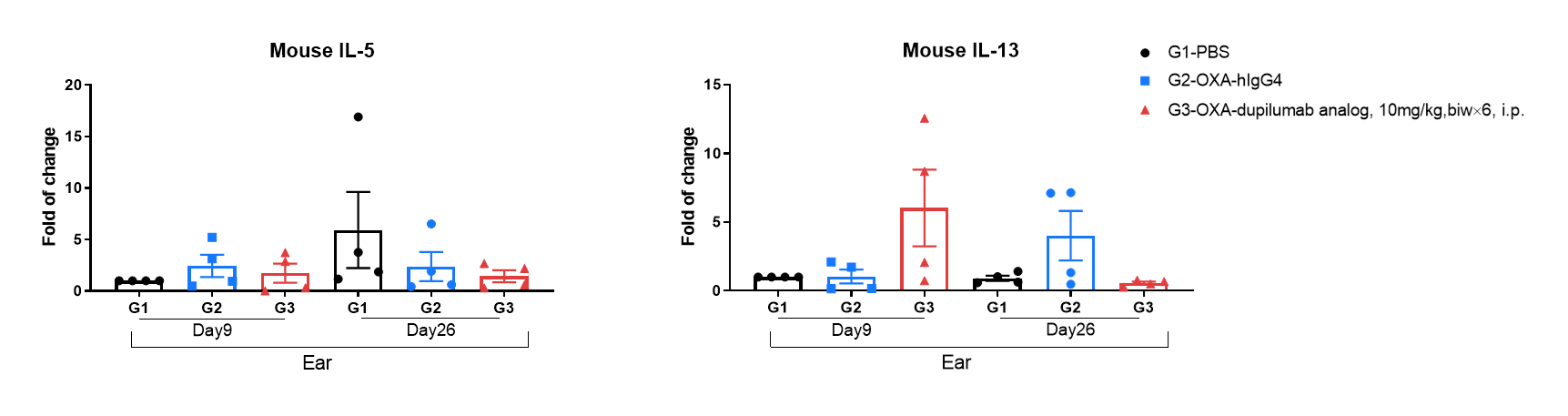
Mouse IL-5 and IL-13 expression analysis in OXA-induced AD model of B-hIL4/hIL4RA mice by RT-qPCR. Ear was collected at day 9 and 26 and total RNA of these samples were extracted. Then RNA samples were reverse transcribed and mouse IL-5, mouse IL-13 levels were measured by RT-qPCR.
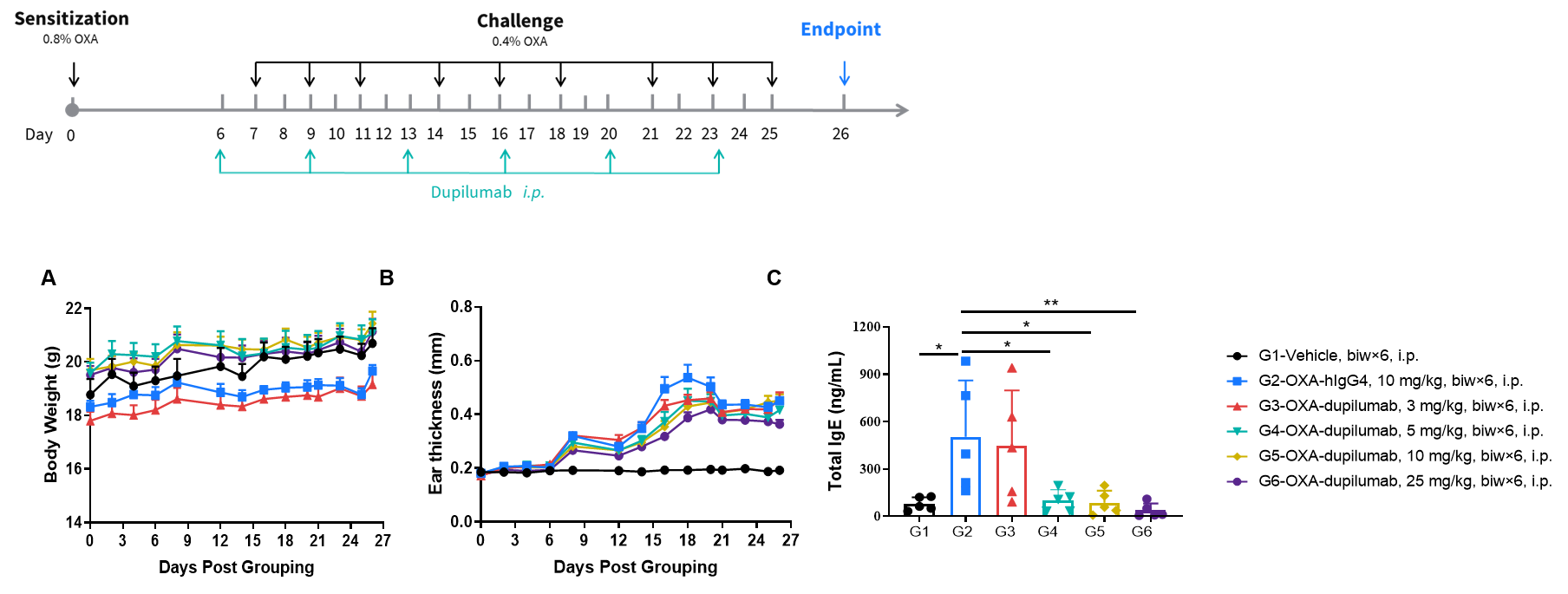
Efficacy of anti-human IL4RA antibody in B-hIL4/hIL4RA mice. Mice in each group were treated with different dose of dupilumab produced in house. Doses are shown in legend. (A) Body weight changes during treatment. (B) Statistical analysis of ear thickness in each group. Epidermis of ear began to desquamate from day 18. So the ear thickness was decreased from day 18 as shown in figure. (C) Total IgE levels in serum. Total IgE levels were measured by ELISA on day 26. Ear thickness and concentrations of total serum IgE were negative related with the doses of antibody. (n = 5).
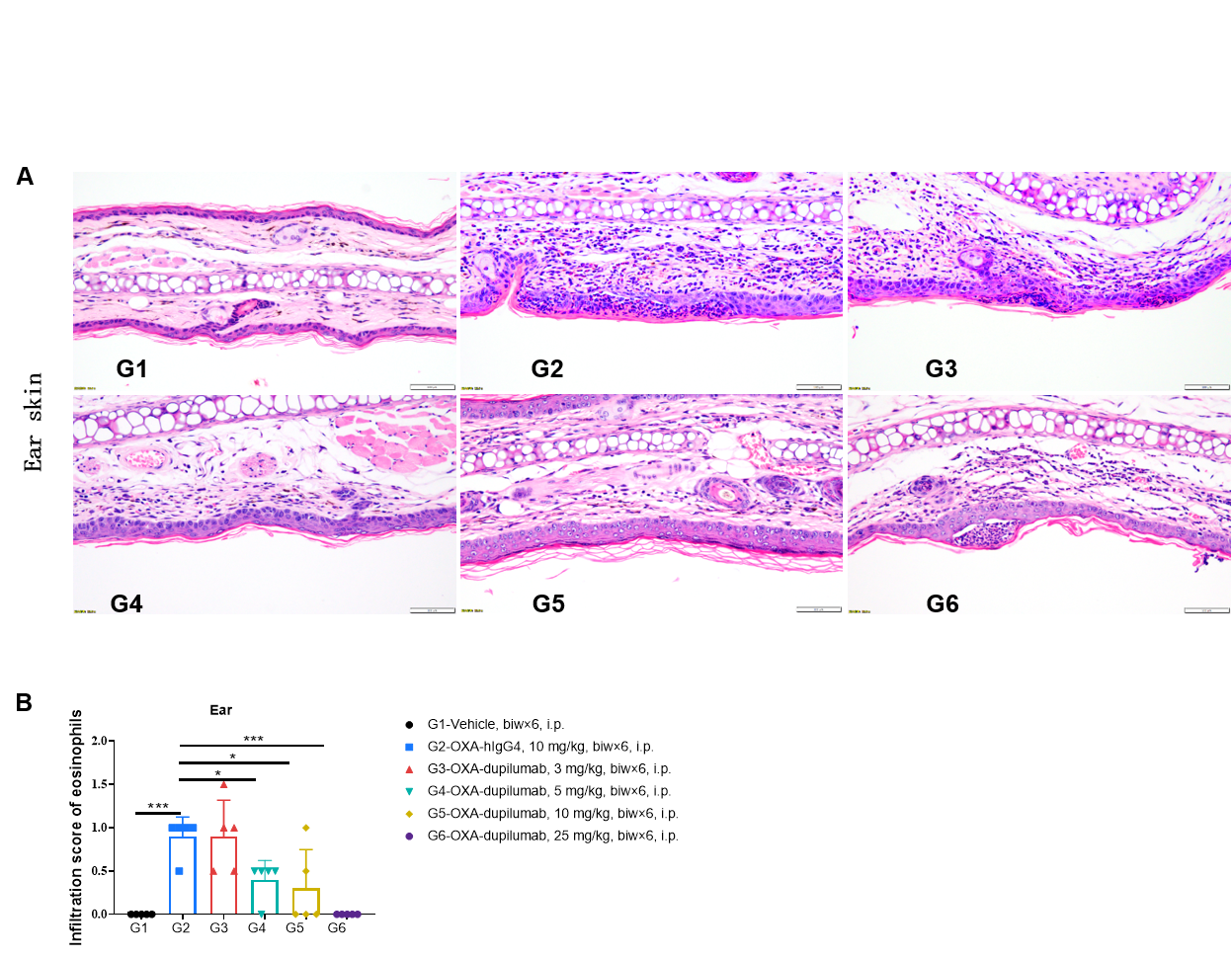
Effects of anti-human IL4RA antibody on inflammatory infiltration in ear skin of the AD mouse model. (A) Hematoxylin and eosin (H&E) staining. (B) Score of eosinophils infiltrated in ear epidermal skin (n=5). Infiltration scores of eosinophils in ear skin were negatively related to the doses of antibody, demonstrating that the B-hIL4/hIL4RA mice provide a powerful preclinical model for in vivo evaluation of anti-human IL4RA antibodies. AD: Atopic dermatitis; ND: Not detectable.
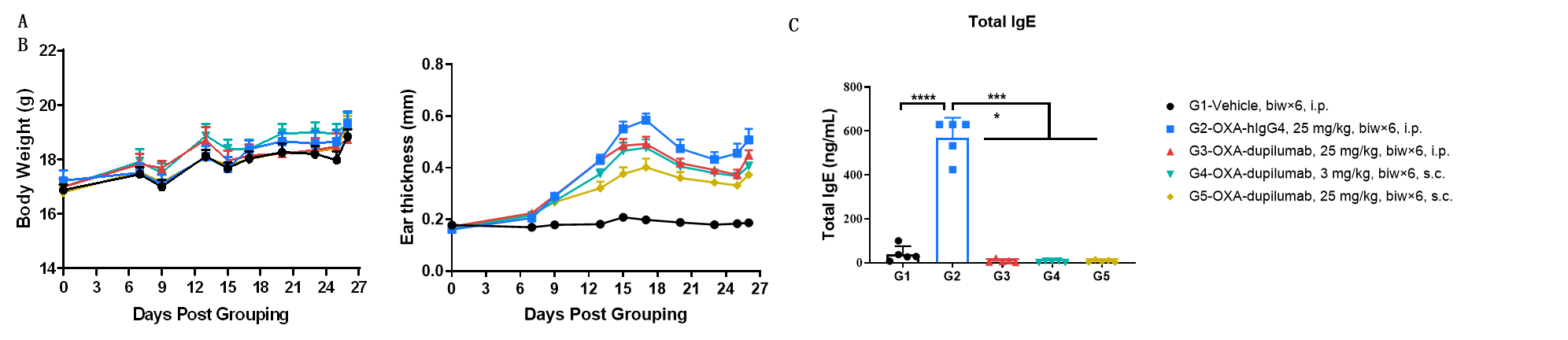
Efficacy of anti-human IL4RA antibody in B-hIL4/hIL4RA mice. Mice in each group were treated with dupilumab (in house) produced in house. Doses are shown in legend. (A) Body weight changes during treatment. (B) Statistical analysis of ear thickness in each group. Epidermis of ear began to desquamate from day 18. So the ear thickness was decreased from day 18 as shown in figure. (C) Total IgE levels in serum. Total IgE levels were measured by ELISA on day 26. Ear thickness and concentrations of total serum IgE were negative related with the doses of antibody. (n = 5).
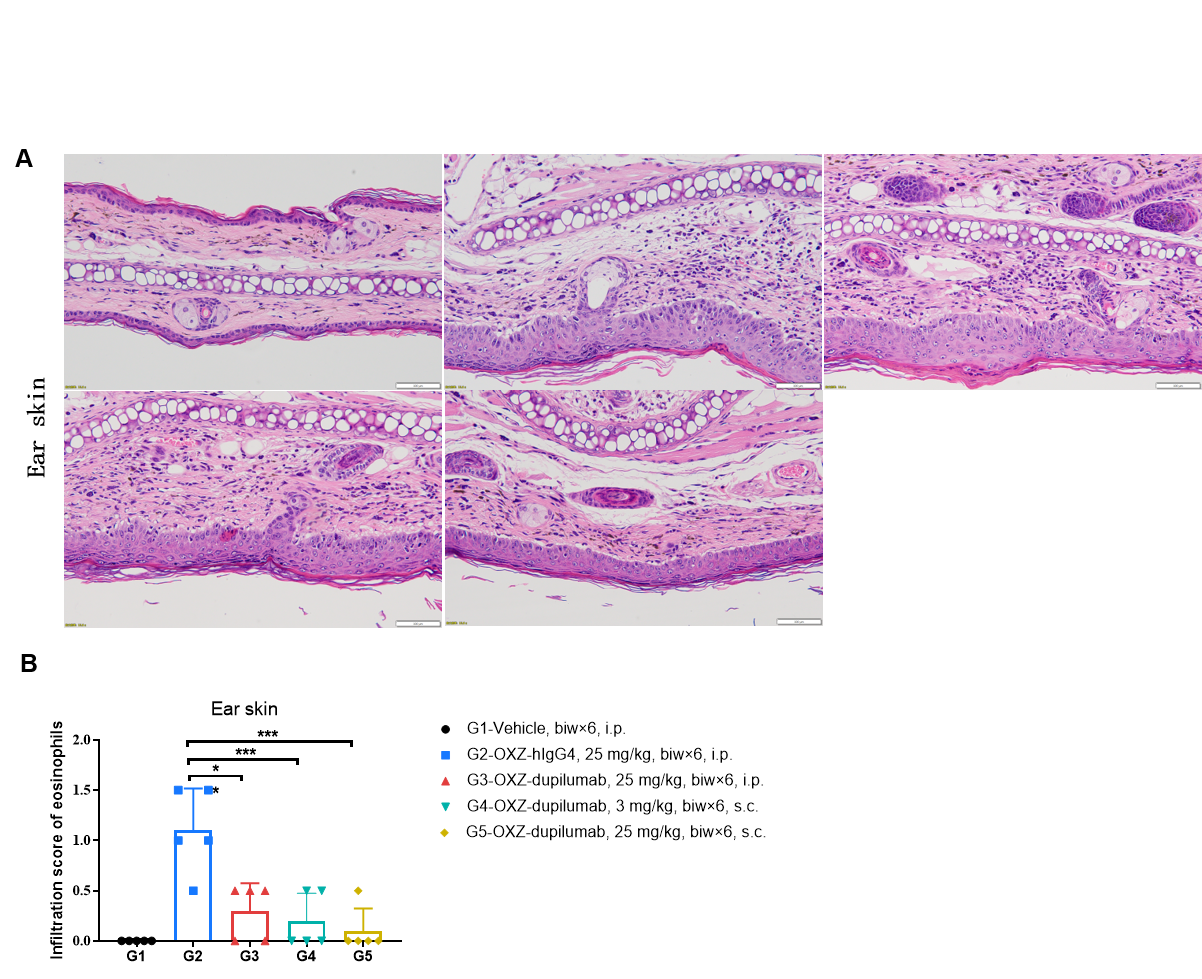
Effects of anti-human IL4RA antibody on inflammatory infiltration in ear skin of the AD mouse model. (A) Hematoxylin and eosin (H&E) staining. (B) Score of eosinophils infiltrated in ear epidermal skin (n=5). Infiltration scores of eosinophils in ear skin were significantly reduced after administration of the antibodies, demonstrating that the B-hIL4/hIL4RA mice provide a powerful preclinical model for in vivo evaluation of anti-human IL4RA antibodies. Infiltration score of eosinophils: 1=slight; 2=mild; 3=moderate; 4=severe. AD: Atopic dermatitis; ND: Not detectable.