
C57BL/6-Pdcd1tm1(PDCD1)Bcgen/Bcgen • 110003
PD-1 (Programmed cell death protein 1, PDCD1, CD279) is an inhibitory receptor expressed on activated T cells, B cells, and myeloid cells. By binding to its ligands PD-L1 and PD-L2, PD-1 plays a critical role in immune checkpoint regulation, suppressing T cell activation and maintaining peripheral tolerance. Dysregulated PD-1 signaling contributes to tumor immune evasion and chronic infection.
In PD-1 humanized mice, the murine Pdcd1 gene is replaced with the human PDCD1 gene, ensuring human-specific PD-1 expression under physiological regulation. These mice exhibit normal immune cell development while allowing precise evaluation of anti-human PD-1 therapeutic antibodies in vivo.
PD-1 humanized mice are designed for in vivo PD-1 antibody validation, checkpoint inhibitor development, and preclinical cancer immunotherapy studies. This model bridges translational gaps by expressing human PD-1 under endogenous regulation.
Key Advantages
Validation
Application
PD-1 humanized mice are designed for in vivo PD-1 antibody validation, checkpoint inhibitor development, and preclinical cancer immunotherapy studies. This model bridges translational gaps by expressing human PD-1 under endogenous regulation.
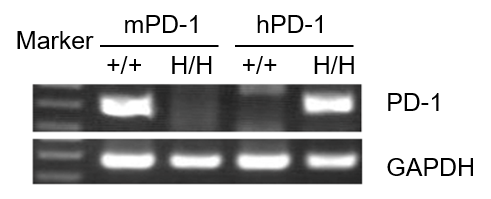
Strain specific analysis of PD-1 gene expression in WT and B-hPD-1 mice by RT-PCR. Mouse PD-1 mRNA was detectable only in splenocytes of wild-type C57BL/6 mice (+/+). Human PD-1 mRNA was detected only in homozygous B-hPD-1 mice, but not in wild-type mice.
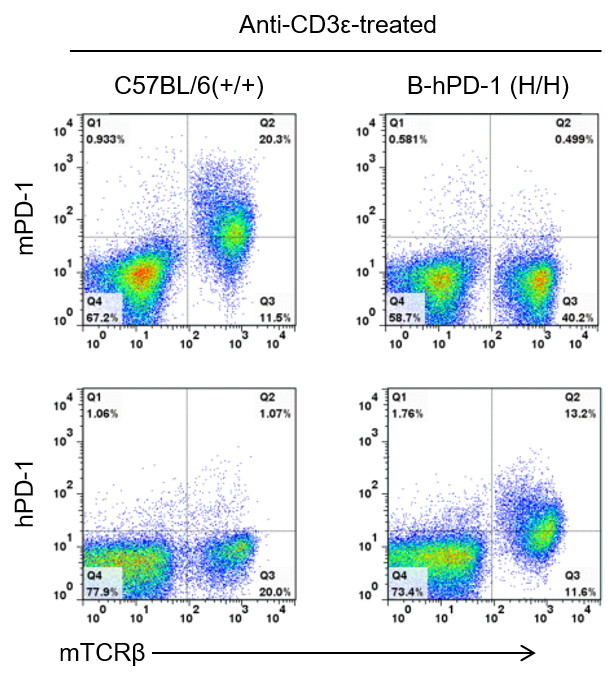
Strain specific PD-1 expression analysis in homozygous B-hPD-1 mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice and homozygous B-hPD-1 mice (H/H) stimulated with anti-CD3ε in vivo, and analyzed by flow cytometry with species-specific anti-PD-1 antibody. Mouse PD-1 was detectable in wild-type mice. Human PD-1 was exclusively detectable in homozygous B-hPD-1 mice but not in wild-type mice.
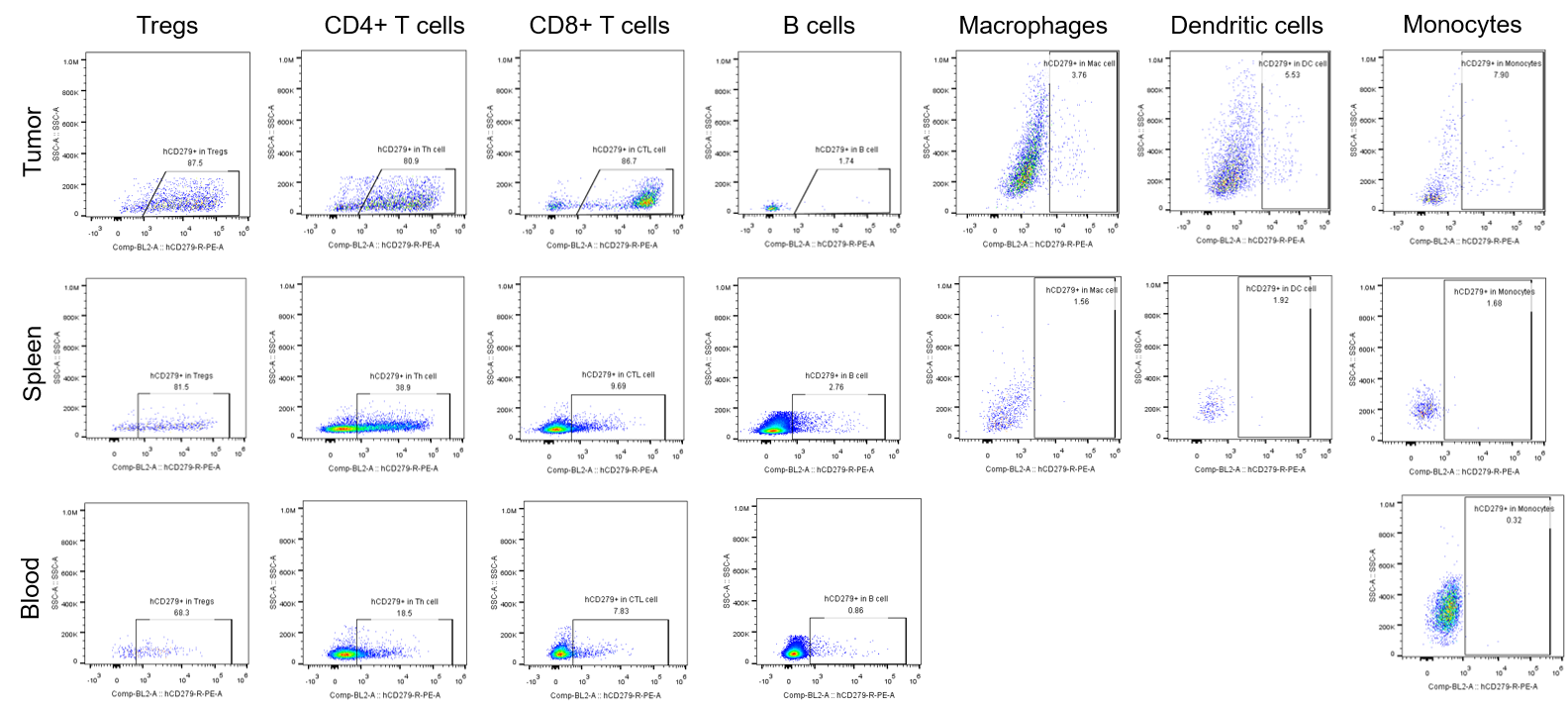
Percentages of human PD-1+ cells in tumor, spleen and blood of B-hPD-1 mice implanted with MC38 cell line. Murine colon cancer MC38 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (female, 8-9-week-old, n=8).Tumor tissue, splenocytes and blood cells were collected 28 days after implantation. Percentages of human PD-1+ cells in Tregs, CD4+ T cells, CD8+ T cells, B cells, macrophages, dendritic cells and monocytes.
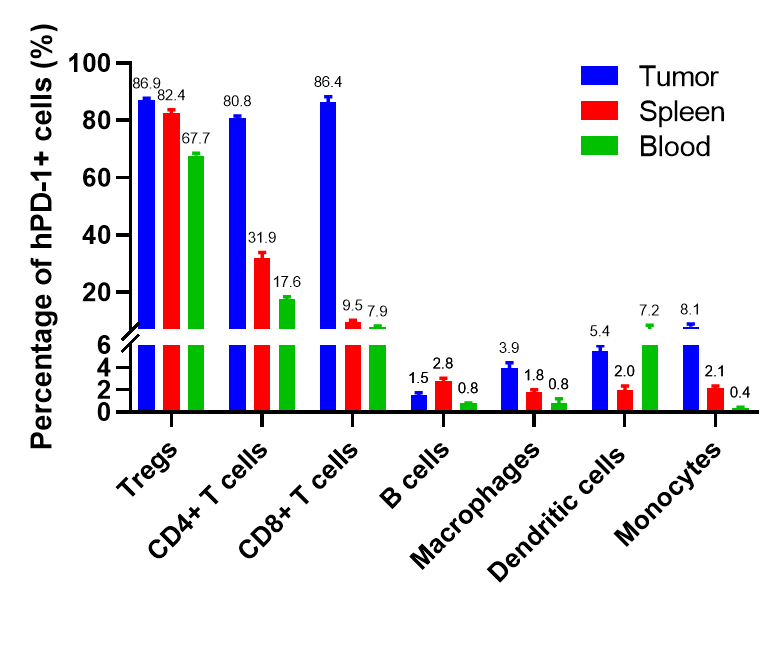
Percentages of human PD-1+ cells in tumor, spleen and blood of B-hPD-1 mice implanted with MC38 cell line. Murine colon cancer MC38 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (female, 8-9-week-old, n=8).Tumor tissue, splenocytes and blood cells were collected 28 days after implantation. Frequency of human PD-1+ cells in Tregs, CD4+ T cells, CD8+ T cells, B cells, macrophages, dendritic cells and monocytes. Values are expressed as mean ± SEM.
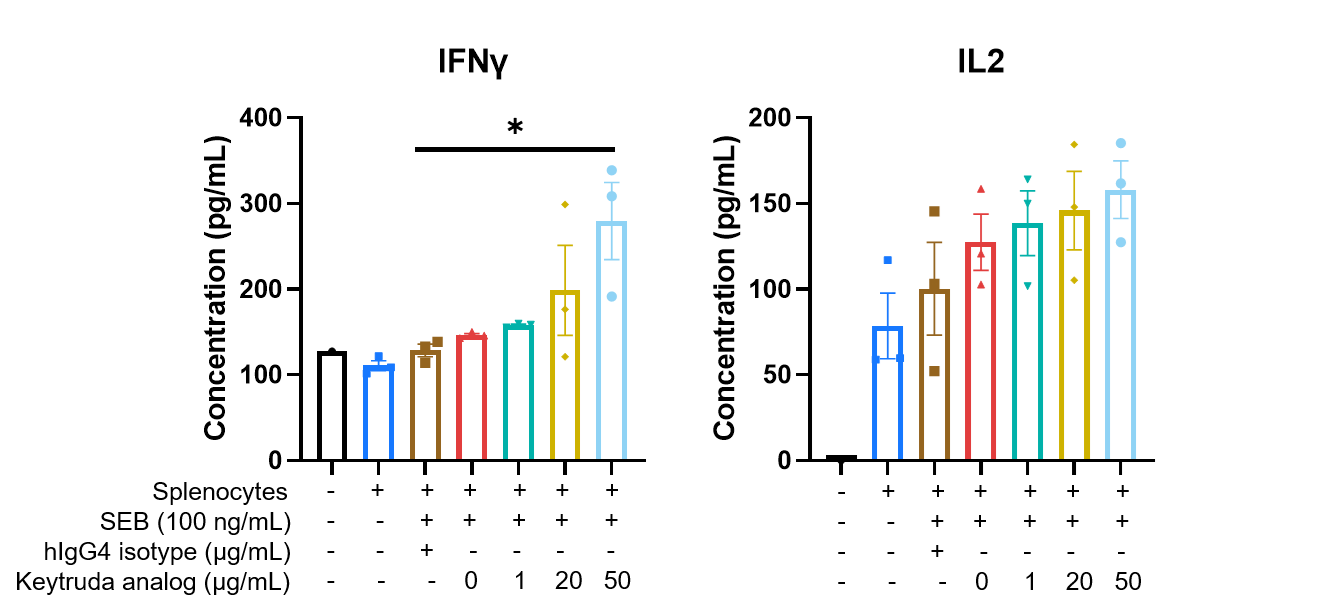
Keytruda analog (in-house) activated T cells in vitro in a dose-dependent manner. Splenocytes were isolated from B-hPD-1 mice and incubated with SEB and Keytruda analog for 72 hrs. Supernatants were collected for detecting the concentration of mouse IFNγ (Cisbio, 62MIFNGPEG) and IL2 (Cisbio, 62MIL02PEG) by HTRF (Homogeneous Time-Resolved Fluorescence) method. The results showed that the addition of keytruda analog increased the secretion of IFNγ and IL2, and this increase was positively correlated with the dosage of the drug (n=3).
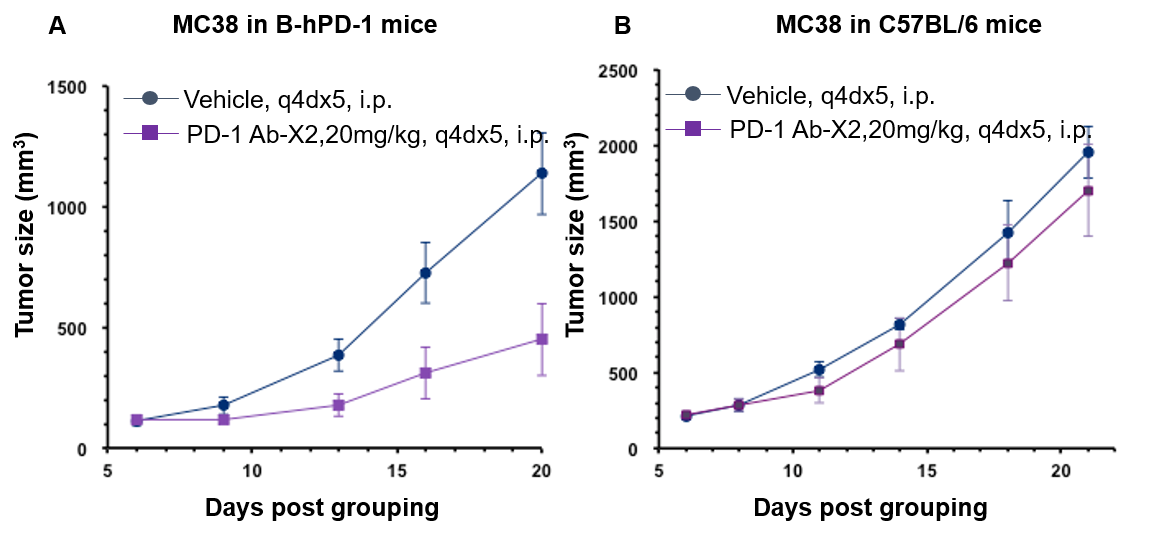
Antitumor activity of anti-human PD-1 antibody in B-hPD-1 mice and C57BL/6 mice. Anti-human PD-1 antibody inhibited MC38 tumor growth in B-hPD-1 mice. (A) Murine colon cancer MC38 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (female, 6 week-old, n=6). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human PD-1 antibody and schedules indicated in panel A. (B) Murine colon cancer MC38 cells (5×105) were subcutaneously implanted into wild-type C57BL/6 mice (female, 6 week-old, n=6). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human PD-1 antibody and schedules indicated in panel B. Results showed that human PD-1 antibody X2 was efficacious in controlling tumor growth in the homozygous B-hPD-1 mice but not in the wild-type C57BL/6 mice, demonstrating that B-hPD-1 mice provide a powerful preclinical model for in vivo evaluation of anti-human PD-1 antibodies. Values are expressed as mean ± SEM.
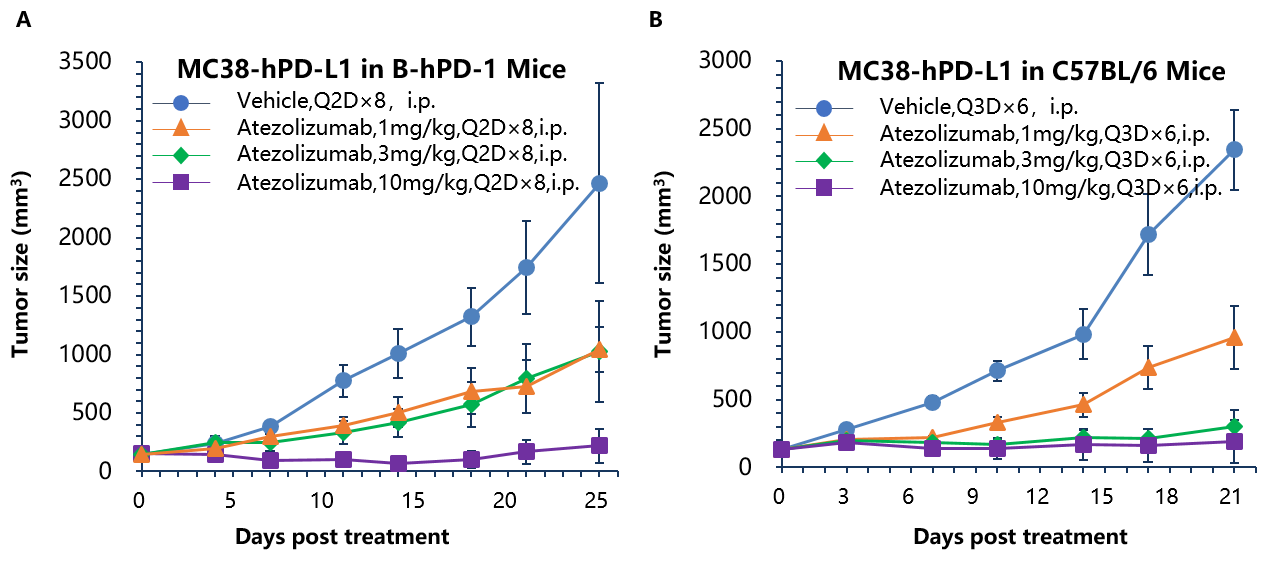
Antitumor activity of anti-human PD-L1 antibody in B-hPD-1 and C57BL/6 mice. Anti-human PD-L1 antibody atezolizumab inhibited MC38-hPD-L1 tumor growth in B-hPD-1 mice (A) and wild type C57BL/6 mice (B). Murine colon cancer MC38-hPD-L1 cells (2×105) were subcutaneously implanted into homozygous B-hPD-1 mice and C57BL/6 mice (female, 6-8 week-old, n=6). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human PD-L1 antibody with doses and schedules indicated in panel. Anti-human PD-L1 antibody was efficacious in controlling tumor growth in homozygous B-hPD-1 mice (A) and wild-type C57BL/6 mice (B). Values are expressed as mean ± SEM.
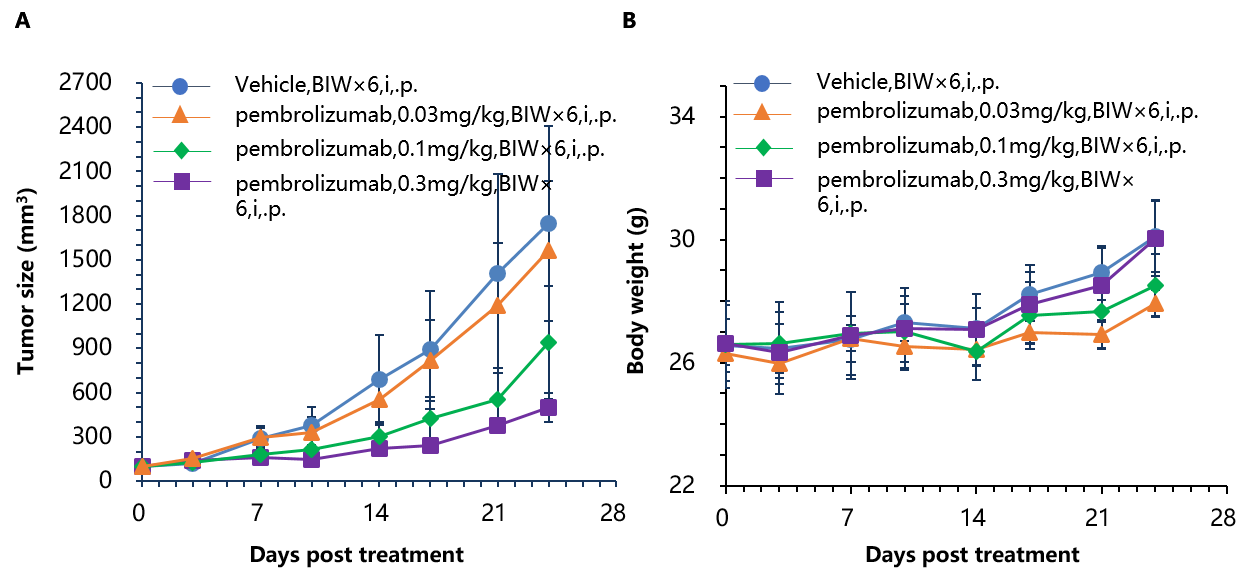
Antitumor activity of anti-human PD-1 antibody in B-hPD-1 mice. (A) Anti-human PD-1 antibody inhibited MC38 tumor growth in B-hPD-1 mice. Murine colon cancer MC38 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (male, 4-6 week-old, n=5). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human PD-1 antibody with doses and schedules indicated in panel (A). (B) Body weight changes during treatment. As shown in panel A, anti-human PD-1 antibody was efficacious in controlling tumor growth in B-hPD-1 mice, demonstrating that B-hPD-1 mice provide a powerful preclinical model for in vivo evaluation of anti-human PD-1 antibodies. Values are expressed as mean ± SEM.
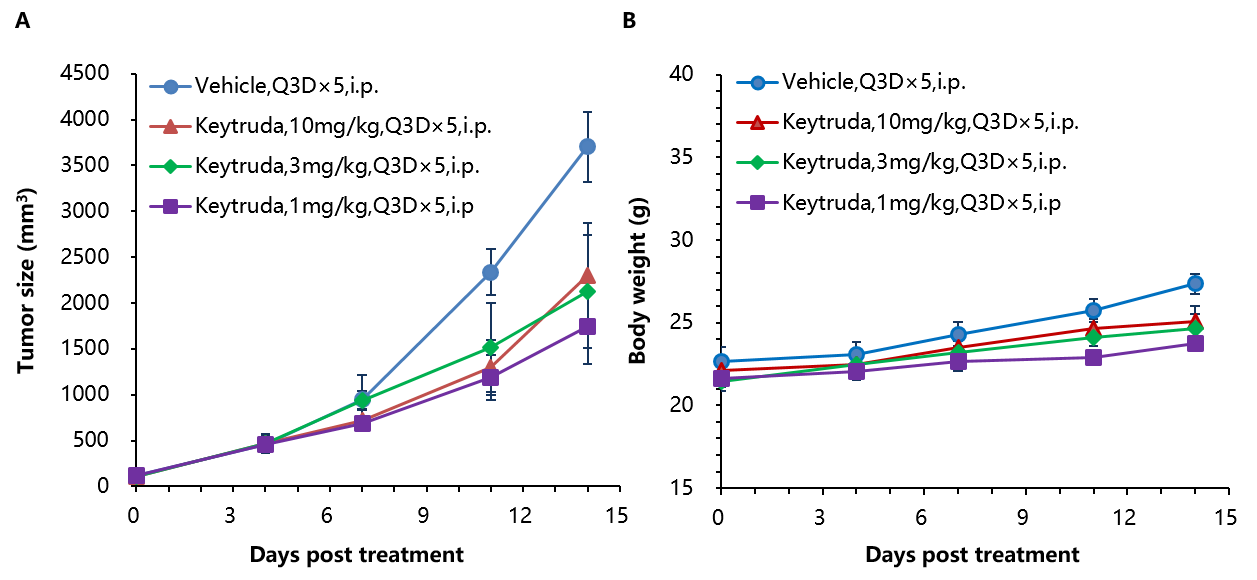
Antitumor activity of anti-human PD-1 antibody in B-hPD-1 mice. (A) Anti-human PD-1 antibody inhibited EL4 tumor growth in B-hPD-1 mice. Murine lymphoma cancer EL4 cells were subcutaneously implanted into homozygous B-hPD-1 mice (n=5). Mice were grouped when tumor volume reached approximately 150±50 mm3, at which time they were treated with three anti-human PD-1 antibodies with doses and schedules indicated in panel. (B) Body weight changes during treatment. As shown in panel A, anti-human PD-1 antibodies were efficacious in controlling tumor growth in B-hPD-1 mice, demonstrating that the B-hPD-1 mice provide a powerful preclinical model for in vivo evaluation of anti-human PD-1 antibodies. Values are expressed as mean ± SEM.
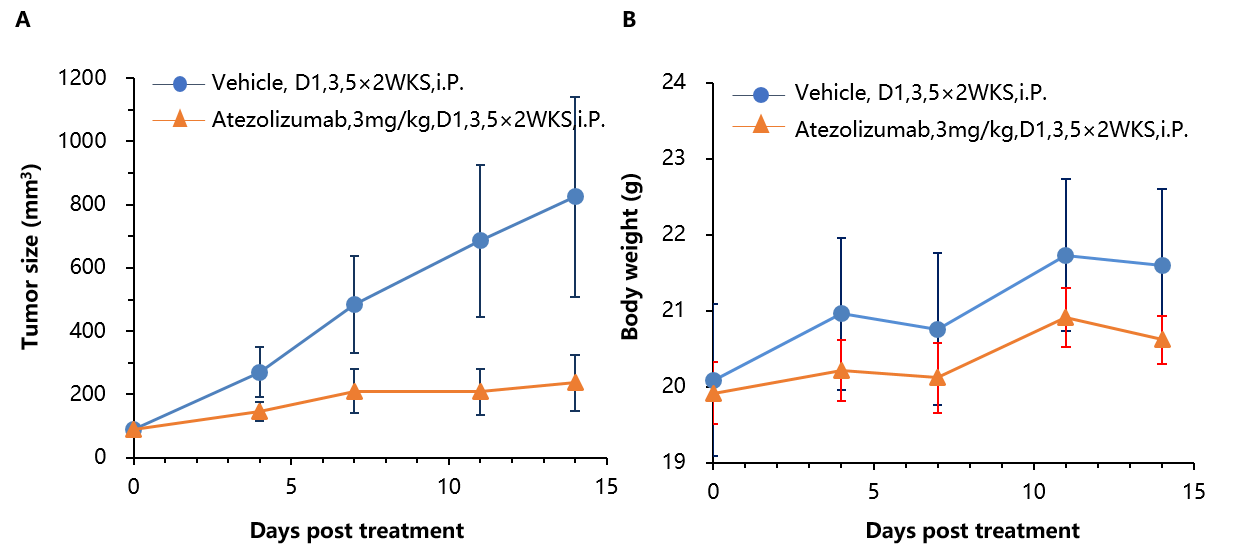
Antitumor activity of anti-human PD-L1 antibody (Atezolizumab) in B-hPD-1 mice. (A) Anti-human PD-L1 antibody inhibited MC38-hPD-L1 tumor growth in B-hPD-1 mice. Murine colon cancer MC38-hPD-L1 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (n=7). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human PD-L1 antibody with doses and schedules indicated in panel A. (B) Body weight changes during treatment. As shown in panel A, anti-human PD-L1 antibody was efficacious in controlling tumor growth in B-hPD-1 mice, demonstrating that the B-hPD-1 mice provide a powerful preclinical model for in vivo evaluation of anti-human PD-L1 antibodies. Values are expressed as mean ± SEM.
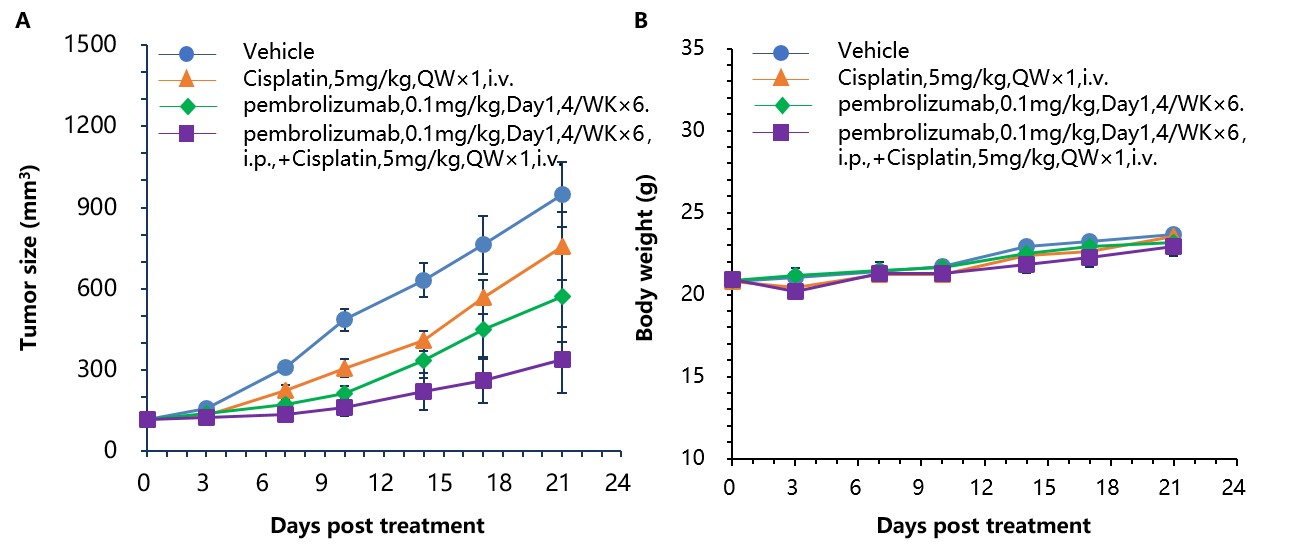
Antitumor activity of anti-human PD-1 antibody combined with cisplatin in B-hPD-1 mice. (A) Anti-human PD-1 antibody combined with cisplatin inhibited MC38-hPD-L1 tumor growth in B-hPD-1 mice. Murine colon cancer MC38-hPD-L1 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (female, 5-8 week-old, n=8). Mice were grouped when tumor volume reached approximately 150±50 mm3, at which time they were treated with anti-human PD-1 antibodies and cisplatin with doses and schedules indicated in panel. (B) Body weight changes during treatment. As shown in panel A, combination of anti-hPD-1 antibody and the chemotherapy drug cisplatin shows more efficaciously inhibitory effects than individual groups, demonstrating that the B-hPD-1 mice provide a powerful preclinical model for in vivo evaluating combination therapy efficacy of anti-human PD-1 antibodies and chemotherapy drugs. Values are expressed as mean ± SEM.
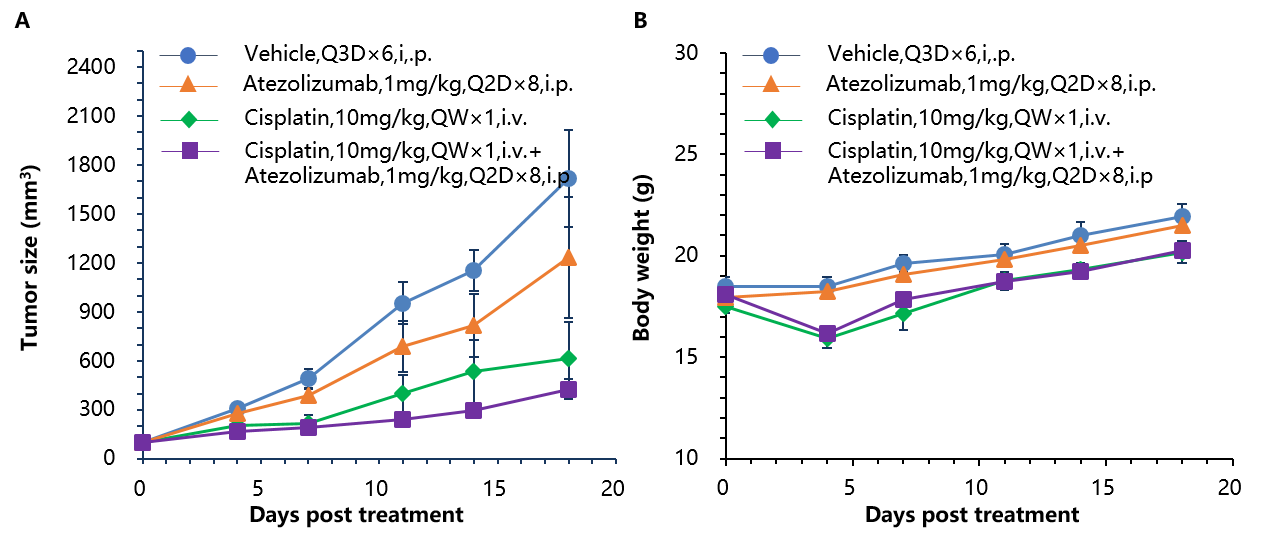
Antitumor activity of anti-human PD-L1 antibody (Atezolizumab) combined with cisplatin in B-hPD-1 mice. (A) Anti-human PD-L1 antibody combined with cisplatin inhibited MC38-hPD-L1 tumor growth in B-hPD-1 mice. Murine colon cancer MC38-hPD-L1 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice (female, 5-8 week-old, n=8). Mice were grouped when tumor volume reached approximately 150±50 mm3, at which time they were treated with anti-human PD-L1 antibody and cisplatin with doses and schedules indicated in panel. (B) Body weight changes during treatment. As shown in panel A, combination of anti-hPD-L1 antibody and the chemotherapy drug cisplatin shows more efficaciously inhibitory effects than individual groups, demonstrating that the B-hPD-1 mice provide a powerful preclinical model for in vivo evaluating combination therapy efficacy of anti-human PD-L1 antibodies and chemotherapy drugs. Values are expressed as mean ± SEM.