
C57BL/6-Tnfsf15tm2(TNFSF15)Bcgen Il23atm1(IL23A)BcgenIl12btm1(IL12B)Bcgen/Bcgen • 113038

| Product name | B-hTL1A/hIL23A/hIL12B mice |
|---|---|
| Catalog number | 113038 |
| Strain name | C57BL/6-Tnfsf15tm2(TNFSF15)Bcgen Il23atm1(IL23A)BcgenIl12btm1(IL12B)Bcgen/Bcgen |
| Strain background | C57BL/6 |
| NCBI gene ID | 3593,51561,9966 (Human) |
| Aliases | CLMF; CLMF2; IL-12B; IMD28; IMD29; NKSF; NKSF2; IL-23; IL-23A; IL23P19; P19; SGRF; TL1; TL1A; TNLG1B; VEGI; VEGI192A; VEGI192A; IL-23; SGRF; CLMF |
| Application | This product is used for pharmacological and safety evaluation of autoimmune diseases such as Inflammatory bowel disease, psoriasis and arthritis. |
Key Advantages
Application
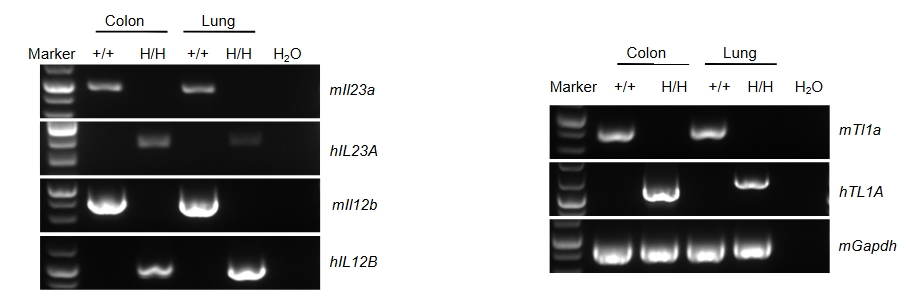
Species-specific analysis of TL1A, IL23A and IL12B gene expression in wild-type C57BL/6 mice and homozygous TL1A/IL23A/IL12B humanized mice was performed by RT-PCR. Colon and lung tissues were collected from wild-type mice C57BL/6 (+/+) and homozygous humanized mice (H/H). Mouse Tl1a, Il23a and Il12b mRNA were detectable only in wild-type mice, whereas human TL1A, IL-23A and IL-12B transcripts were detectable only in homozygous TL1A/IL23A/IL12B humanized mice.
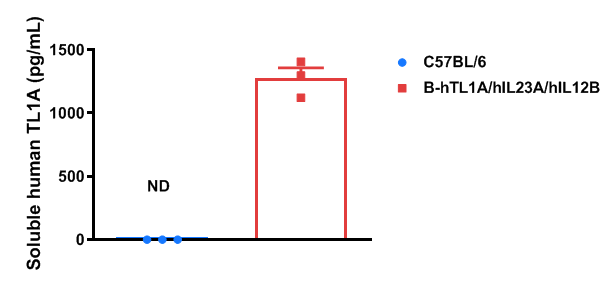
Soluble TL1A expression analysis in TL1A/IL23A/IL12B humanized mice was performed by ELISA. Bone marrow-derived dendritic cells from wild-type and humanized mice (female, 9-week-old, n = 3) were stimulated with LPS. Supernatants were collected and analyzed for soluble TL1A. Human TL1A was exclusively detectable in TL1A/IL23A/IL12B humanized mice; mouse TL1A was detectable only in wild-type mice. Values are expressed as mean ± SEM. ND = not detectable.
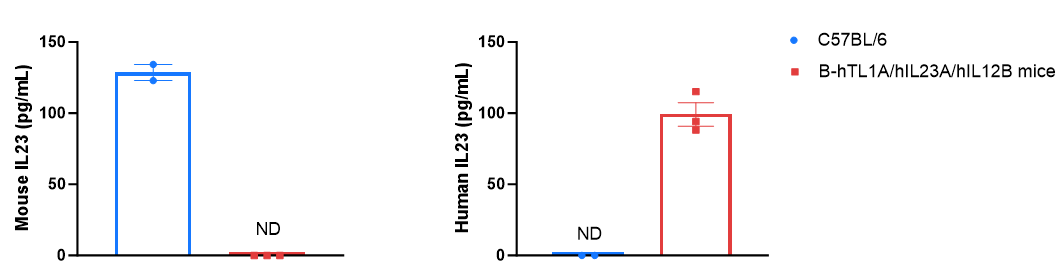
Strain-specific IL-23 expression analysis in TL1A/IL23A/IL12B humanized mice. Bone marrow-derived dendritic cells were produced by culturing the bone marrow from wild-type C57BL/6 mice and homozygous B-hTL1A/hIL23A/hIL12B mice (female, 6-week-old, n=3), which were stimulated with LPS in vitro. After stimulation, the supernatants were collected, and the levels of mouse and human IL-23 were analyzed by ELISA. Mouse IL-23 was only detectable in wild-type C57BL/6 mice, whereas human IL-23 was exclusively detectable in homozygous TL1A/IL23A/IL12B humanized mice. Values are expressed as mean ± SEM. ND = not detectable.
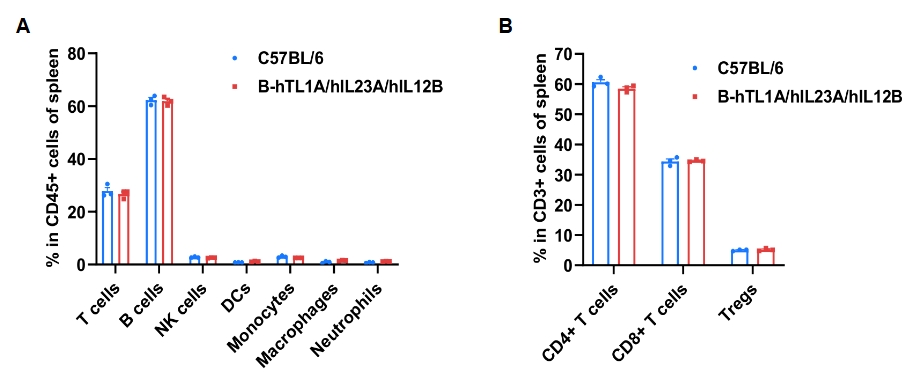
Frequency of leukocyte subpopulations in spleen was analyzed by flow cytometry. Splenocytes were isolated from wild-type and TL1A/IL23A/IL12B humanized mice (female, 7-week-old, n = 3). Flow cytometry analysis was performed to quantify leukocyte subsets and T-cell subpopulations. Percentages of T cells, B cells, NK cells, DCs, monocytes, macrophages, neutrophils, CD4⁺ T cells, CD8⁺ T cells and Tregs in humanized mice were comparable to wild-type mice. Values are expressed as mean ± SEM.
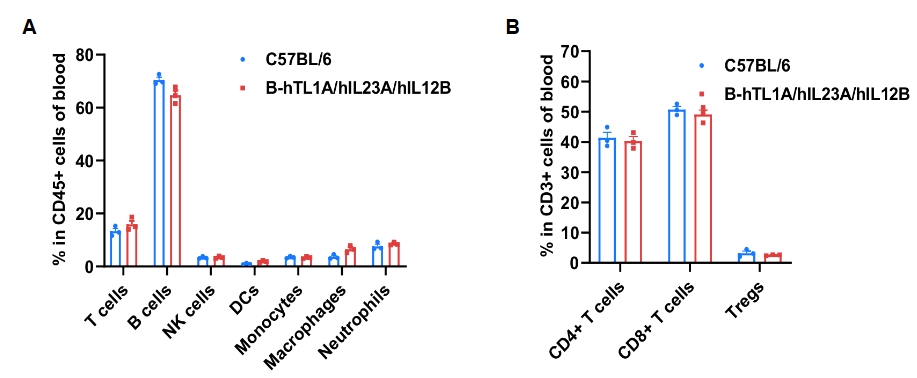
Blood leukocyte subpopulation profiling was performed via flow cytometry using samples from wild-type and TL1A/IL23A/IL12B humanized mice (female, 7-week-old, n = 3). Leukocyte and T-cell subset frequencies (CD4⁺, CD8⁺ and Tregs) were similar between groups, indicating that humanization of TL1A, IL23A and IL12B does not change the frequency or distribution of these cell types in the blood. Values are expressed as mean ± SEM.
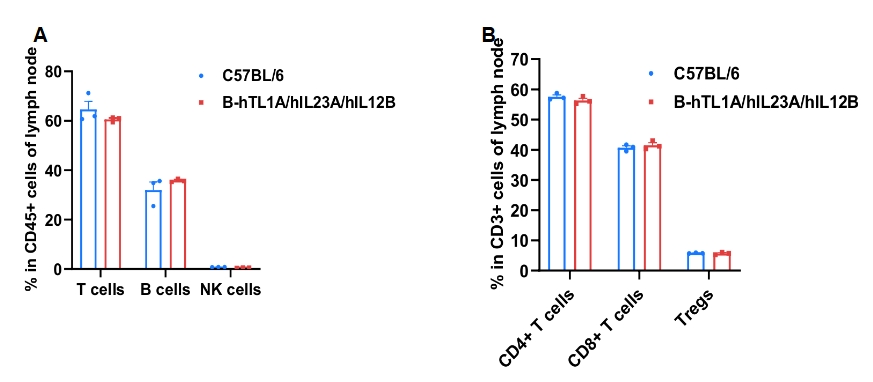
Lymph-node leukocyte profiling was performed using samples from wild-type and TL1A/IL23A/IL12B humanized mice (female, 7-week-old, n = 3). Frequencies of T cells, B cells, NK cells, CD4⁺ T cells, CD8⁺ T cells and Tregs were comparable between genotypes, indicating that humanization of TL1A, IL23A and IL12B does not change the frequency or distribution of these cell types in lymph nodes. Values are expressed as mean ± SEM.
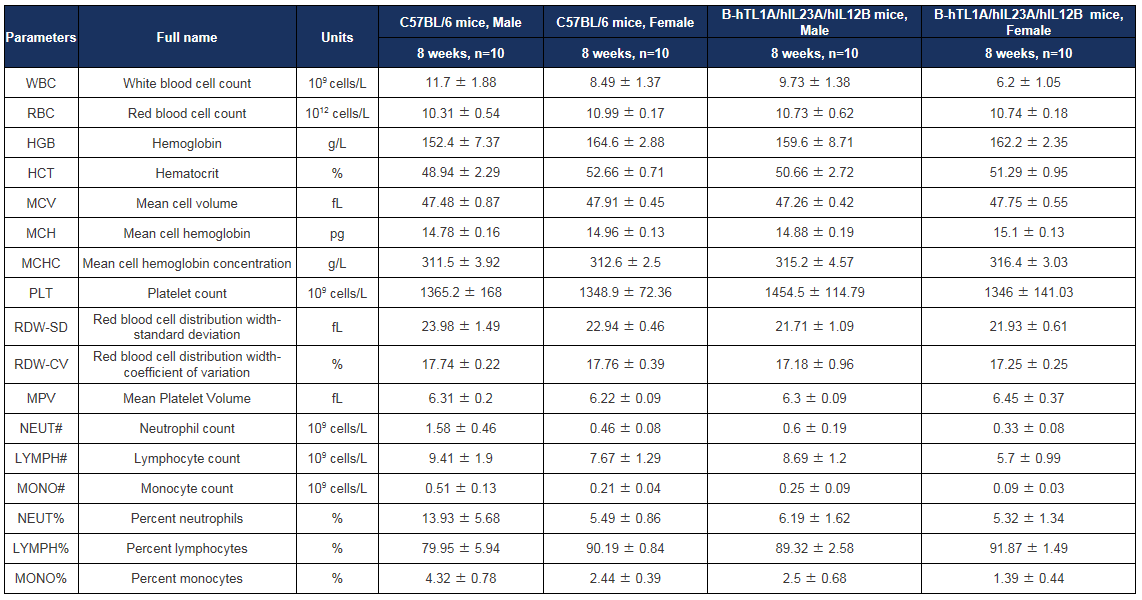
Complete blood count (CBC) of TL1A/IL23A/IL12B humanized mice. All parameters were within normal physiological ranges. Values are expressed as mean ± SD.
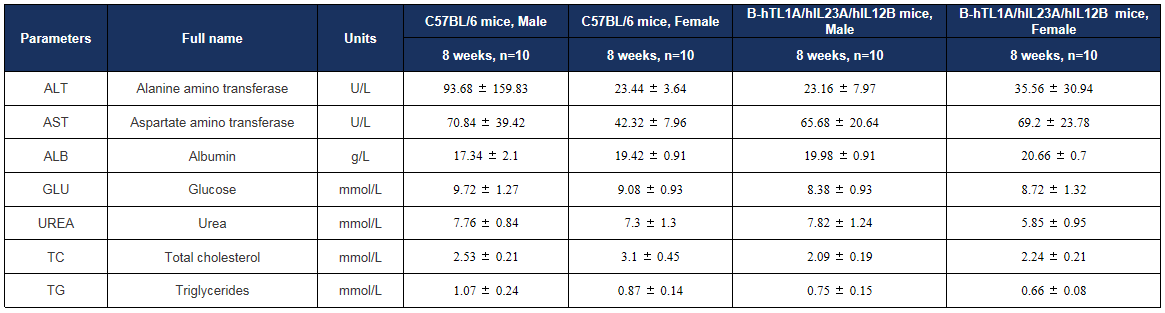
Serum biochemistry of TL1A/IL23A/IL12B humanized mice. Blood biochemical markers remained comparable to wild-type levels, indicating stable systemic physiology. Values are expressed as mean ± SD.
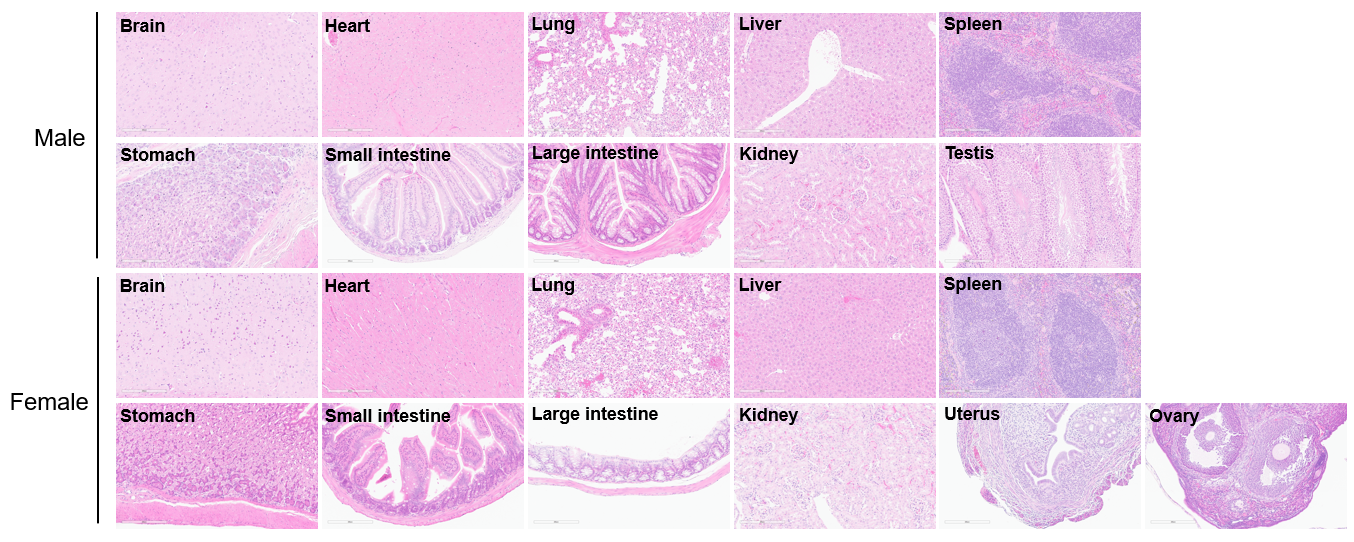
Histopathological examination of major organs from TL1A/IL23A/IL12B humanized mice at 32 weeks of age (male, n = 3; female, n = 3). H&E staining of brain, heart, lung, liver, spleen, stomach, small intestine, large intestine, kidney, testis, uterus and ovary revealed no obvious abnormalities, demonstrating good long-term safety.
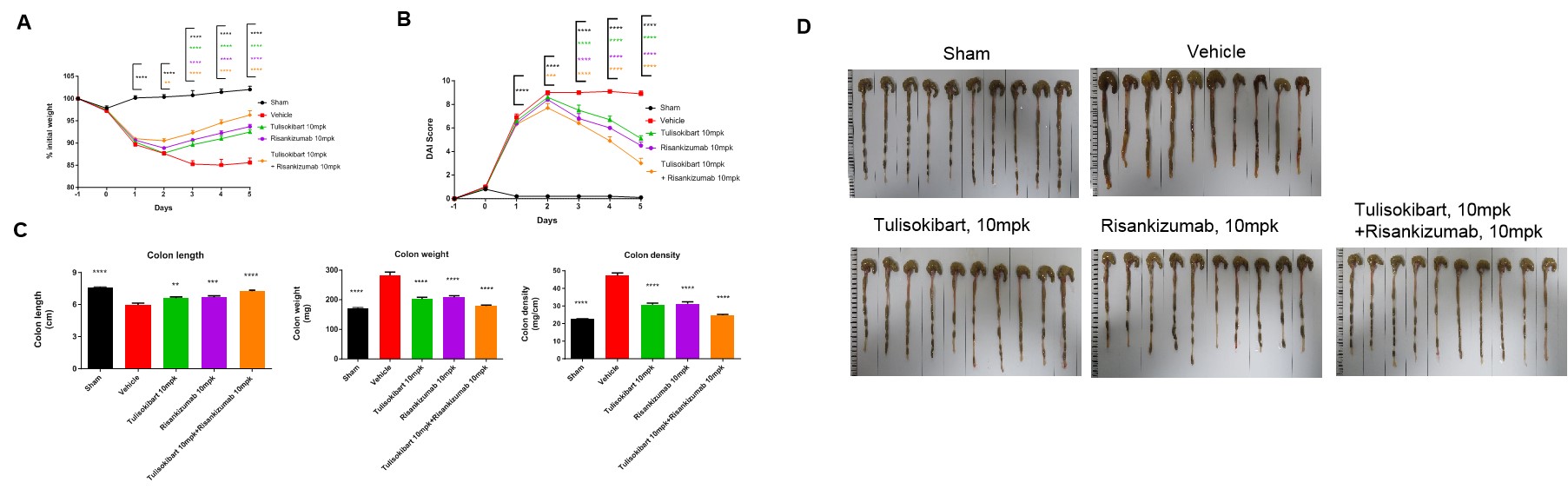
TNBS solution was instilled into the colonic lumen of TL1A/IL23A/IL12B humanized mice (female, 8–10 weeks old, n = 10). Sham controls received 50% ethanol. Treatment groups received anti-human TL1A antibody Tulisokibart (10 mpk, WuXi AppTec) and anti-human IL-23p19 antibody Risankizumab (10 mpk, WuXi AppTec), administered alone or in combination. Body weight and DAI were recorded daily. On day 5, mice were sacrificed and colon length and weight were measured.(A) Body-weight change. (B) DAI score. (C) Colon index. (D) Colon images. The TNBS model was successfully established. Both antibodies significantly alleviated acute colitis, and combination therapy showed superior efficacy. TL1A/IL23A/IL12B humanized mice constitute a robust model for evaluating anti-human TL1A and anti-human IL-23p19 antibodies in vivo. Values are expressed as mean ± SEM.*p < 0.05, **p < 0.01, ***p < 0.001, ***p < 0.0001 vs vehicle, ANOVA.
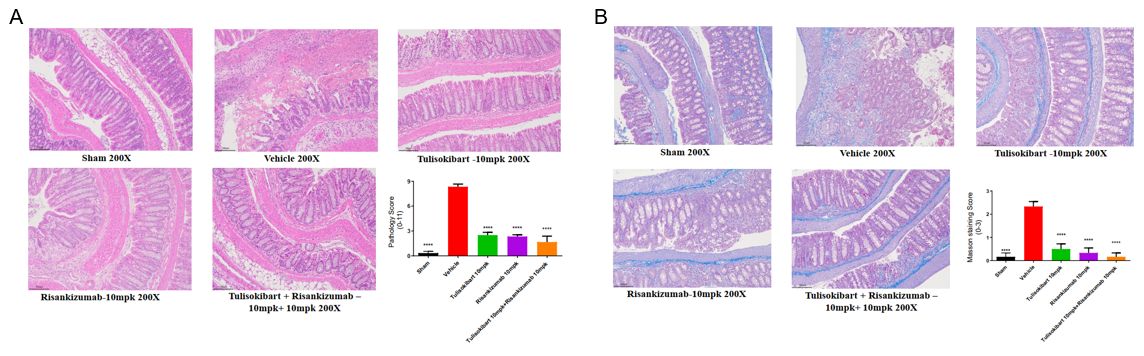
TNBS-induced colonic pathology and fibrosis in TL1A/IL23A/IL12B humanized mice. Using the same dosing regimen, colon tissues were collected on day 5 for H&E and Masson staining. (A) Pathological score. (B) Masson fibrosis score. Both antibodies significantly reduced epithelial damage and fibrotic progression, with combination therapy providing enhanced protection. Values are expressed as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs Vehicle, ANOVA.
Note: Study conducted by WuXi AppTec.
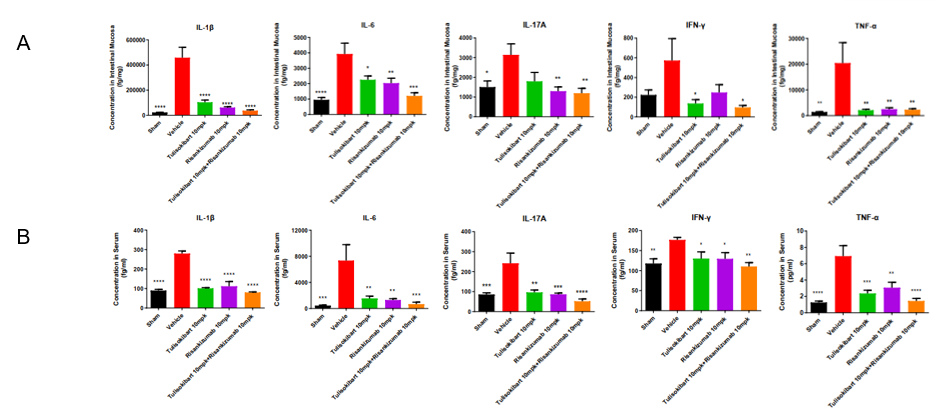
Cytokine analysis in TNBS-induced acute colitis in TL1A/IL23A/IL12B humanized mice. Following the same treatment protocol, mucosa and serum were collected on day 5 for cytokine assessment. (A) IL-1β, IL-6, IL-17A, IFN-γ, TNF-α in intestinal mucosa. (B) IL-1β, IL-6, IL-17A, IFN-γ, TNF-α in serum. Anti-human TL1A and anti-human IL-23p19 antibodies significantly reduced inflammatory cytokine levels, and combination therapy provided the strongest inhibition, highlighting the model’s utility for preclinical testing of human-specific TL1A/IL23 pathway therapeutics. Values are expressed as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ***p<0.0001 vs Vehicle, ANOVA.
Note: Study conducted by WuXi AppTec.
Q1. What are TL1A/IL23A/IL12B humanized mice?
A triple-cytokine humanized strain expressing human TL1A, IL-23A and IL-12B for IBD and colitis research.
Q2. What studies are these mice best suited for?
TNBS-induced colitis, cytokine biology, fibrosis, immune pathway analysis, and evaluation of anti-human TL1A, IL-23, and IL-12B antibodies.
Q3. Do these mice maintain normal immune homeostasis?
Yes. Leukocyte and T-cell distributions in spleen, blood and lymph nodes remain comparable to wild-type mice.
Q4. Can human-specific antibodies be directly tested?
Yes. Anti-human TL1A and anti-human IL-23p19 antibodies were tested and showed robust in vivo efficacy in this model.
Q5. Are these mice suitable for combination therapy studies?
Yes. The model supports evaluation of combination therapy targeting TL1A and IL-23 pathways.