
C57BL/6-Cd3etm1(CD3E)Bcgen Cd3dtm1(CD3D)Bcgen Cd3gtm1(CD3G)Bcgen/Bcgen • 110039

| Product name | B-hCD3EDG mice |
|---|---|
| Catalog number | 110039 |
| Strain name | C57BL/6-Cd3etm1(CD3E)Bcgen Cd3dtm1(CD3D)Bcgen Cd3gtm1(CD3G)Bcgen/Bcgen |
| Strain background | C57BL/6 |
| NCBI gene ID | 916,915,917 (Human) |
| Aliases | T3E; TCRE; IMD18; CD3epsilon; T3D; IMD19; CD3DELTA; CD3-DELTA; T3G; IMD17; CD3GAMMA; CD3-GAMMA |
The B-hCD3EDG humanized mouse model was developed by replacing the mouse Cd3e/d/g genes with their human counterparts, resulting in expression of a chimeric human CD3E–CD3D–CD3G (CD3EDG) complex while knocking out the endogenous mouse genes. Expression studies confirmed that human CD3E, CD3D, and CD3G mRNA and protein are exclusively detectable in homozygous B-hCD3EDG mice, whereas mouse CD3 proteins are present only in wild-type controls.
Immune profiling across spleen, blood, lymph nodes, and thymus demonstrated that humanization does not alter immune cell composition, as T cells, B cells, NK cells, dendritic cells, monocytes, and T-cell subsets (CD4⁺, CD8⁺, Tregs) were comparable to wild-type mice. Functional assays further validated that T cells from B-hCD3EDG mice proliferate and secrete cytokines upon anti-human CD3E stimulation in vitro, confirming normal T cell functionality.
Together, these results establish B-hCD3EDG mice as a robust preclinical platform for the in vivo and in vitro evaluation of CD3-targeted therapies, including bispecific antibodies, T cell engagers, and CAR-T cell therapies.

Strain-specific expression of CD3E, CD3D, CD3G was assessed by RT-PCR in wild-type (+/+) and homozygous CD3EDG humanized mice (H/H). Mouse Cd3e, Cd3d, and Cd3g mRNA were detectable only in thymocytes of wild-type mice, whereas chimeric human CD3E, CD3D, and CD3G mRNA were exclusively detectable in homozygous CD3E humanized mice. These results confirm strain-specific expression of human CD3EDG in the model.
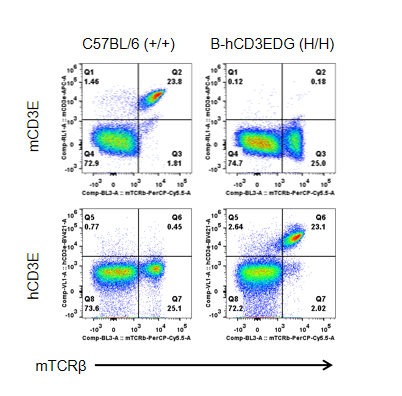
Strain-specific CD3E protein expression analysis in CD3EDG humanized mice.
Splenocytes were collected from wild-type (+/+) and homozygous CD3EDG humanized mice (H/H) and analyzed by flow cytometry using species-specific anti-CD3ε antibodies. Mouse CD3E protein was detectable only in wild-type mice, whereas human CD3E protein was exclusively detectable in homozygous CD3EDG humanized mice. These results confirm strain-specific expression of human CD3E protein in the model.
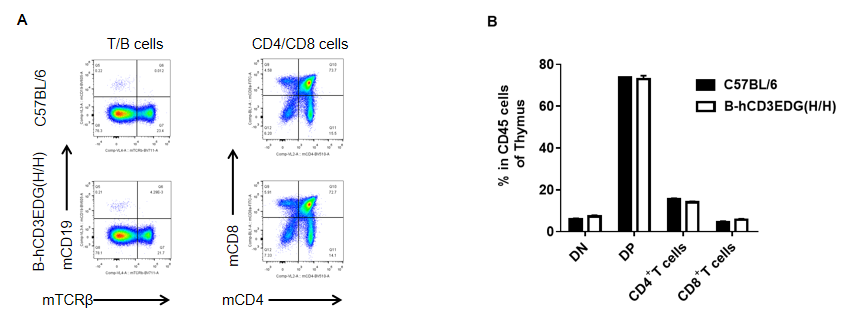
Analysis of thymus leukocyte subpopulations by flow cytometry in CD3EDG humanized mice. Thymocytes were isolated from female wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 7-week-old). Flow cytometry (FACS) was performed to assess thymic leukocyte subpopulations. (A) Representative FACS plots: single live cells were gated for CD45⁺ populations and further analyzed. (B) Quantitative analysis: the proportions of CD4⁺/CD8⁺ T cells and B cells in CD3EDG humanized mice were comparable to wild-type controls, indicating that replacement of mouse Cd3edg with human CD3EDG does not alter thymus immune cell development, differentiation, or distribution.
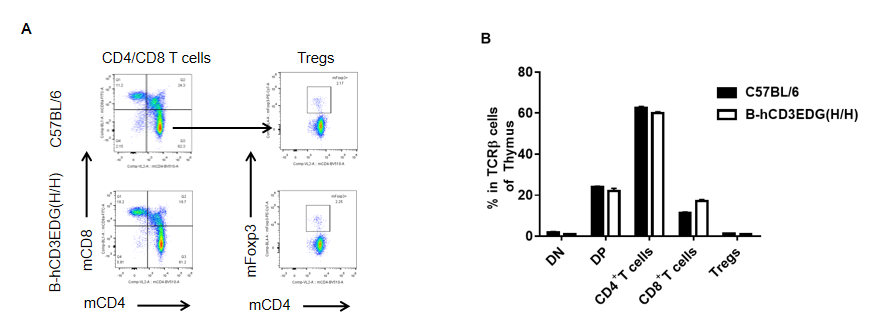
Analysis of thymus T cell subpopulations by flow cytometry in CD3EDG humanized mice. Thymocytes were isolated from female wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 7-week-old). Flow cytometry (FACS) was performed to assess thymic T cell subpopulations. (A) Representative FACS plots: single live CD45⁺ cells were gated for CD3⁺ T cells and further analyzed. (B) Quantitative analysis: the percentages of CD8⁺, CD4⁺, and regulatory T cells (Tregs) in CD3EDG humanized mice were comparable to those in wild-type controls. These results indicate that replacement of mouse Cd3edg with human CD3EDG does not alter thymic T cell development, differentiation, or distribution. Values are expressed as mean ± SEM.
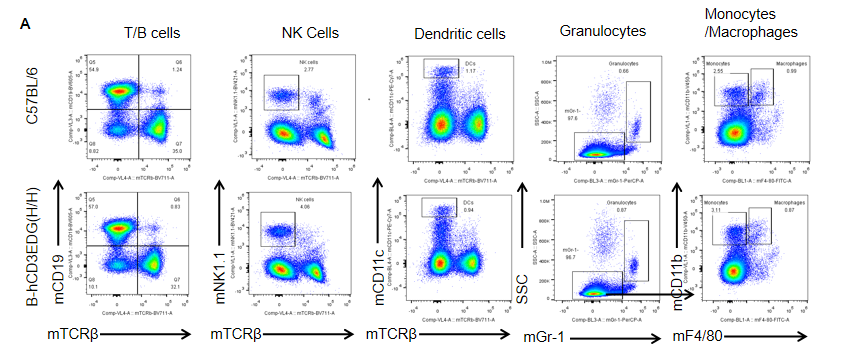
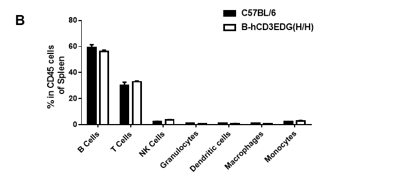
Analysis of spleen leukocyte subpopulations by flow cytometry in CD3EDG humanized mice. Splenocytes were isolated from female wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 7-week-old). Flow cytometry (FACS) was performed to assess splenic leukocyte subpopulations. (A) Representative FACS plots: single live cells were gated for CD45⁺ populations and further analyzed. (B) Quantitative analysis: the percentages of T cells, B cells, NK cells, dendritic cells (DCs), granulocytes, monocytes, and macrophages in CD3EDG humanized mice were comparable to those in wild-type controls. These results indicate that replacing mouse Cd3edg with human CD3EDG does not alter the development, differentiation, or distribution of immune cell subsets in the spleen.
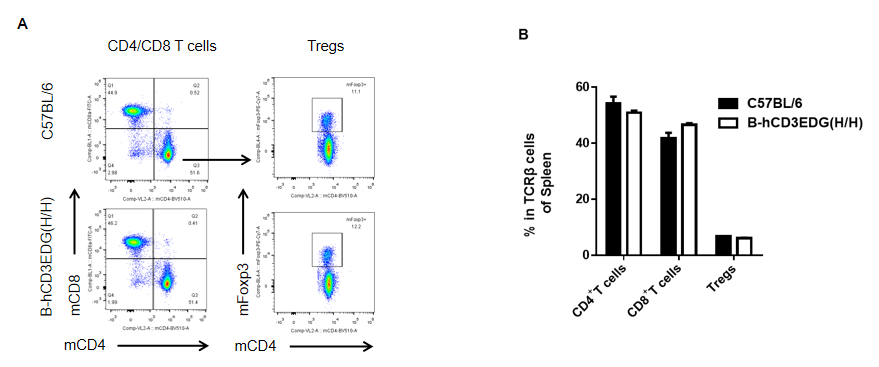
Analysis of spleen T cell subpopulations by flow cytometry in CD3EDG humanized mice. Splenocytes were isolated from female wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 7-week-old). Flow cytometry (FACS) was performed to assess splenic T cell subpopulations. (A) Representative FACS plots: single live CD45⁺ cells were gated for CD3⁺ T cells and further analyzed. (B) Quantitative analysis: the percentages of CD8⁺ T cells, CD4⁺ T cells, and regulatory T cells (Tregs) in CD3EDG humanized mice were comparable to those in wild-type controls. These results indicate that replacing mouse Cd3edg with human CD3EDG does not alter the development, differentiation, or distribution of splenic T cell subsets. Values are expressed as mean ± SEM.
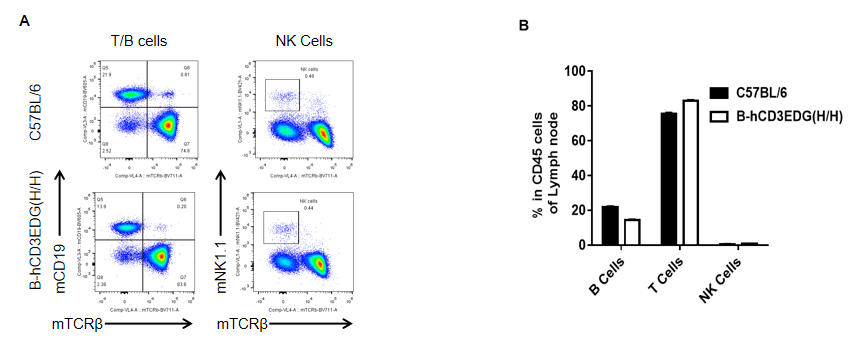
Analysis of lymph node leukocyte subpopulations by flow cytometry in CD3EDG humanized mice. Lymph nodes were isolated from female wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 7-week-old). Flow cytometry (FACS) was performed to assess leukocyte subpopulations. (A) Representative FACS plots: single live CD45⁺ cells were gated and further analyzed. (B) Quantitative analysis: the percentages of T cells, B cells, and NK cells in CD3EDG humanized mice were comparable to those in wild-type controls. These findings demonstrate that replacement of mouse Cd3edg with human CD3EDG does not alter lymph node immune cell development, differentiation, or distribution.
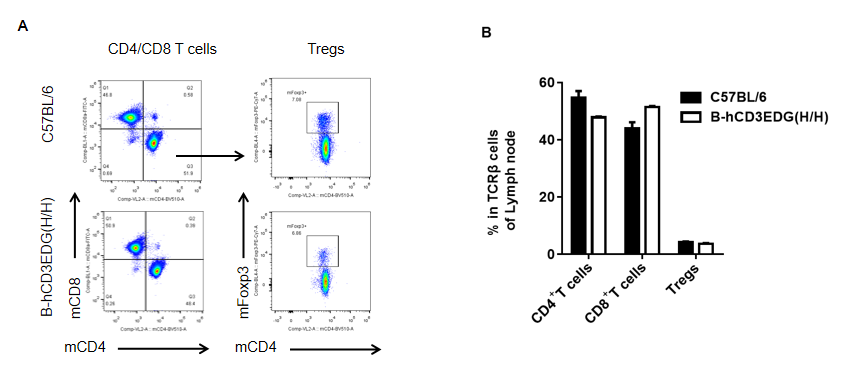
Analysis of lymph node T cell subpopulations by flow cytometry in CD3EDG humanized mice. Lymph nodes were isolated from female wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 7-week-old). Flow cytometry (FACS) was performed to assess lymph node T cell subpopulations. (A) Representative FACS plots: single live CD45⁺ cells were gated for CD3⁺ T cells and further analyzed. (B) Quantitative analysis: the percentages of CD8⁺ T cells, CD4⁺ T cells, and regulatory T cells (Tregs) in CD3EDG humanized mice were comparable to those in wild-type controls. These findings demonstrate that replacement of mouse Cd3edg with human CD3EDG does not alter the development, differentiation, or distribution of T cell subsets in lymph nodes. Values are expressed as mean ± SEM.
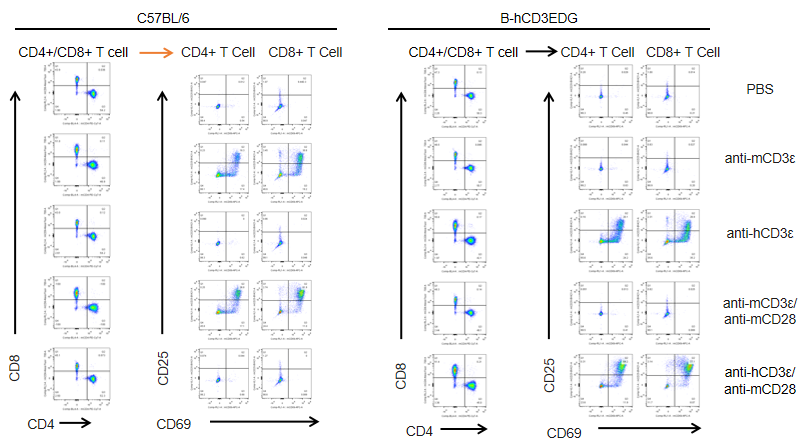
In vitro T cell activation by anti-CD3ε antibody in CD3EDG humanized mice (24h). T cells (2 × 10⁵) were isolated from splenocytes of wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 16-week-old) and incubated with anti-human CD3ε antibody (2 µg/mL) or anti-mouse CD3ε antibody (2 µg/mL) together with anti-mouse CD28 antibody (5 µg/mL) for 24 hours. T cell proliferation was assessed by flow cytometry. T cell activation in CD3EDG humanized mice was significantly upregulated by anti-human CD3ε antibody, at levels comparable to those observed in wild-type mice treated with anti-mouse CD3ε antibody.
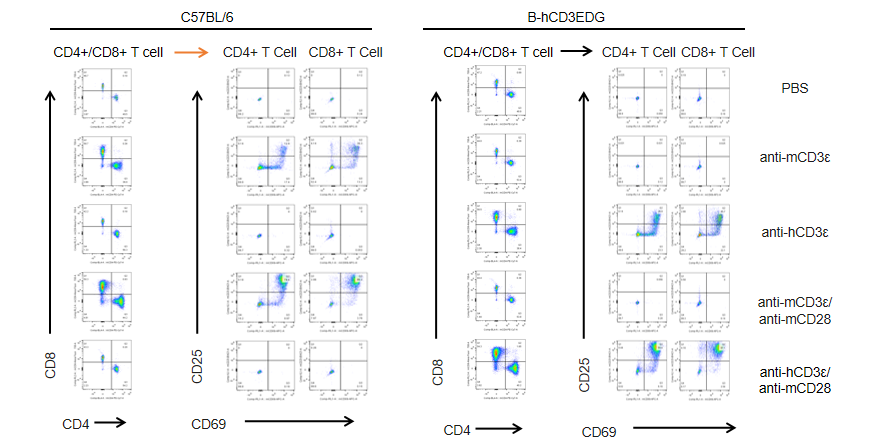
In vitro T cell activation by anti-CD3ε antibody in CD3EDG humanized mice (48h). T cells (2 × 10⁵) were isolated from splenocytes of wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 16-week-old) and incubated with anti-human CD3ε antibody (2 µg/mL) or anti-mouse CD3ε antibody (2 µg/mL) together with anti-mouse CD28 antibody (5 µg/mL) for 48 hours. T cell proliferation was assessed by flow cytometry. T cell activation in CD3EDG humanized mice was significantly upregulated by anti-human CD3ε antibody, at levels comparable to those observed in wild-type mice treated with anti-mouse CD3ε antibody.
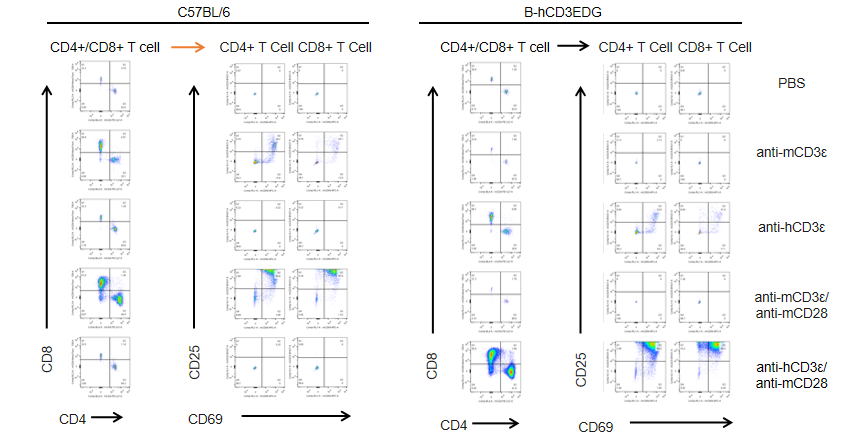
In vitro T cell activation by anti-CD3ε antibody in CD3EDG humanized mice (72h). T cells (2 × 10⁵) were isolated from splenocytes of wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 16-week-old) and incubated with anti-human CD3ε antibody (2 µg/mL) or anti-mouse CD3ε antibody (2 µg/mL) together with anti-mouse CD28 antibody (5 µg/mL) for 72 hours. T cell proliferation was assessed by flow cytometry. T cell activation in CD3EDG humanized mice was significantly upregulated by anti-human CD3ε antibody, at levels comparable to those observed in wild-type mice treated with anti-mouse CD3ε antibody.
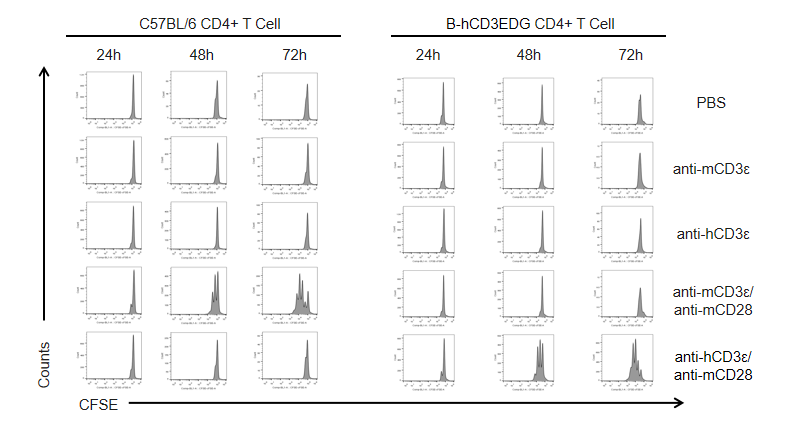
In vitro CD4+ T cell activation kinetics by anti-CD3ε antibody in CD3EDG humanized mice. T cells (2 × 10⁵) were isolated from the splenocytes of wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 16-week-old) and incubated with anti-human CD3ε antibody (2 µg/mL) or anti-mouse CD3ε antibody (2 µg/mL) together with anti-mouse CD28 antibody (5 µg/mL) for 24, 48, and 72 hours. T cell proliferation was measured by flow cytometry. T cell activation in CD3EDG humanized mice was specifically upregulated by anti-human CD3ε antibody, at levels comparable to those observed in wild-type mice treated with anti-mouse CD3ε antibody.
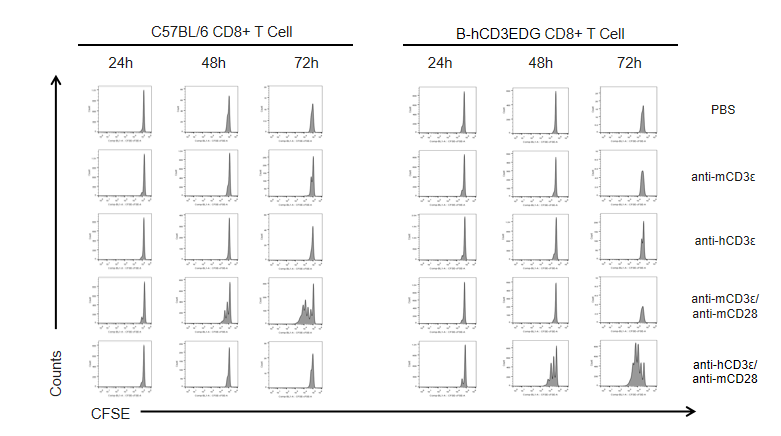
In vitro CD8+ T cell activation kinetics in CD3EDG humanized mice following anti-CD3ε stimulation.
T cells (2 × 10⁵) were isolated from the splenocytes of wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 16-week-old) and incubated with anti-human CD3ε antibody (2 µg/mL) or anti-mouse CD3ε antibody (2 µg/mL) together with anti-mouse CD28 antibody (5 µg/mL) for 24, 48, and 72 hours. T cell proliferation was measured by flow cytometry. T cell activation in CD3EDG humanized mice was specifically upregulated by anti-human CD3ε antibody, at levels comparable to those observed in wild-type mice treated with anti-mouse CD3ε antibody.
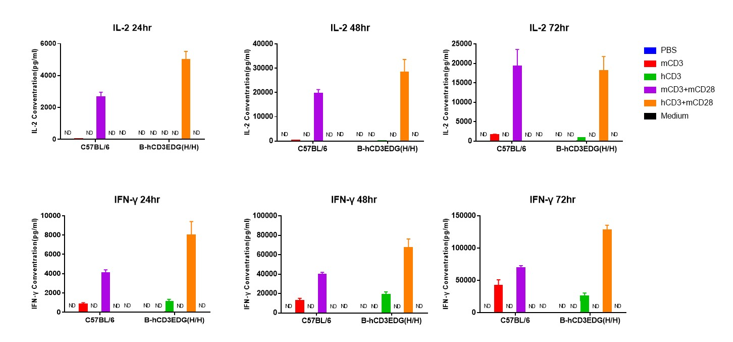
In vitro cytokine production (IFN-γ and IL-2) in CD3EDG humanized mice following anti-CD3ε stimulation. T cells (2 × 10⁵) were isolated from the splenocytes of wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 16-week-old) and incubated with anti-human CD3ε antibody (2 µg/mL) or anti-mouse CD3ε antibody (2 µg/mL) together with anti-mouse CD28 antibody (5 µg/mL) for 24, 48, and 72 hours. IFN-γ and IL-2 production was then measured by ELISA. There was no significant difference in cytokine production between CD3EDG humanized mice and wild-type C57BL/6 mice, indicating that humanization does not impair T cell function. ND: not detectable.
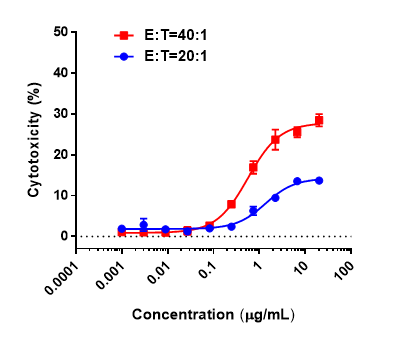
In vitro cytotoxicity of CD3 x BCMA bispecific antibody in CD3EDG humanized mice. Splenocytes from homozygous CD3EDG humanized mice were co-cultured with B-hBCMA MC38 tumor cells and treated with various concentrations of client-provided CD3 x BCMA bispecific antibody. Cytotoxic activity was assessed after 48 hours. When the effector-to-target (E:T) cell ratios were 20:1 and 40:1, killing activity was observed, with a stronger cytotoxic effect detected at the higher 40:1 ratio.
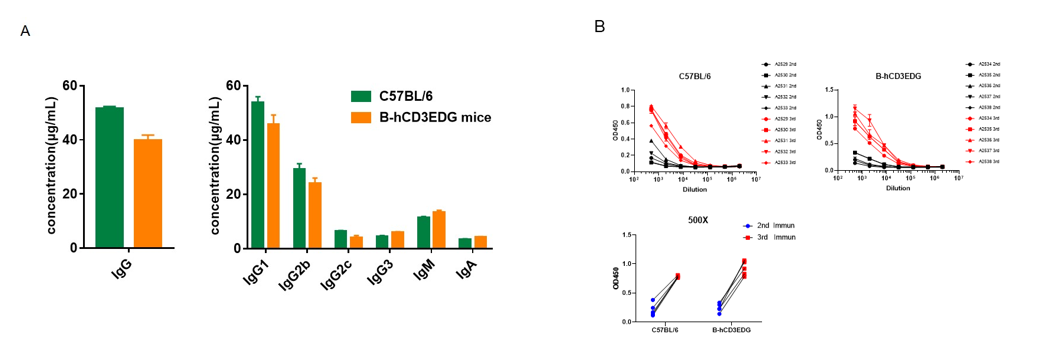
Serum titers of OVA-specific antibodies in CD3EDG humanized mice. Homozygous CD3EDG humanized mice (n=5, 6-week-old) were immunized three times with ovalbumin (OVA) at 2-week intervals. Blood samples were collected one week after each immunization. (A) Quantification of serum immunoglobulin subtypes before immunization. (B) Serum titer analysis after the second and third immunizations. The levels of OVA-specific antibody titers in CD3EDG humanized mice before immunization were similar to those in wild-type C57BL/6 mice. After the third immunization, serum titers of OVA-specific antibodies significantly increased in both strains, demonstrating that replacement of mouse Cd3edg with human CD3EDG does not impair the humoral immune response. Values are expressed as mean ± SEM.
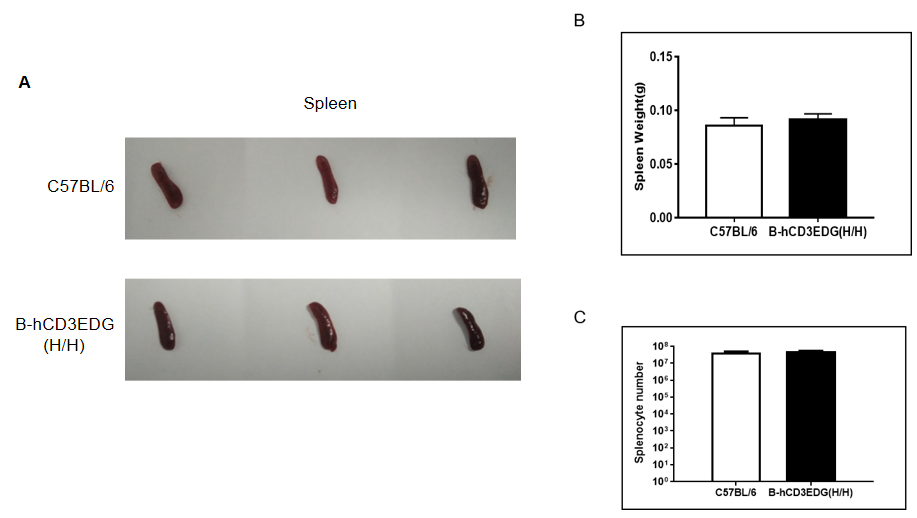
Spleen weight and splenocyte counts in CD3EDG humanized mice. (A) Representative spleen images from wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 7-week-old). (B) Spleen weights were comparable between CD3EDG humanized mice and wild-type controls. (C) Total splenocyte numbers were also similar between the two groups, indicating that replacement of mouse Cd3edg with human CD3EDG does not affect spleen development or splenocyte cellularity.
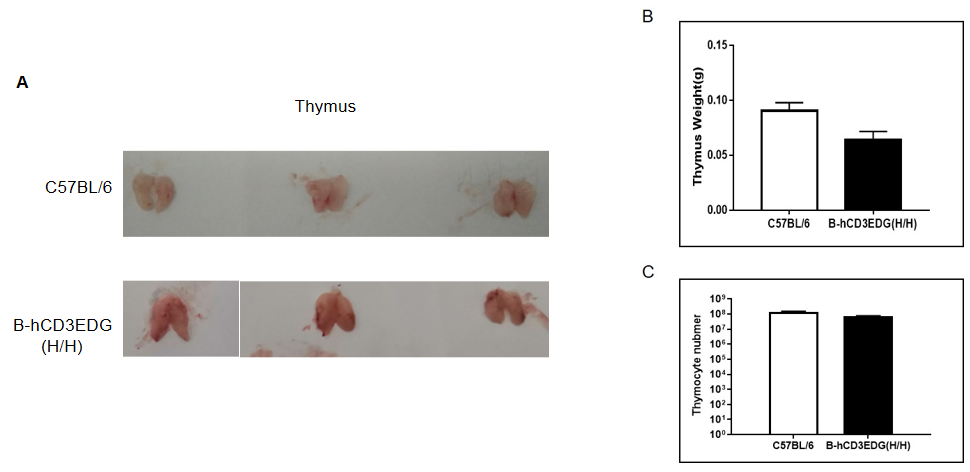
Thymus weight and thymocyte counts in CD3EDG humanized mice. (A) Representative thymus images from wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=3, 7-week-old). (B) Thymus weight in CD3EDG humanized mice was lower than that in wild-type controls. (C) Similarly, total thymocyte numbers in CD3EDG humanized mice were reduced compared with wild-type mice, indicating that replacement of mouse Cd3edg with human CD3EDG affects thymus size and cellularity.
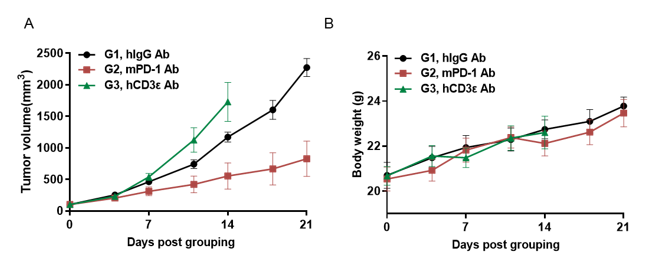
Antitumor activity of anti-hCD3ε antibody and anti-mPD-1 antibody in CD3EDG humanized mice.
Murine colon cancer MC38 cells were subcutaneously implanted into female homozygous CD3EDG humanized mice (8-week-old, n=5). Mice were grouped when tumor volume reached approximately 100 ± 50 mm³ and treated with antibodies at the doses and schedules indicated. (A) Tumor volume changes during treatment. (B) Body weight changes during treatment. Anti-mPD-1 antibody significantly inhibited tumor growth in CD3EDG humanized mice, confirming normal T cell function. In contrast, tumor growth accelerated after anti-hCD3ε antibody treatment, likely due to activation-induced cell death (AICD). These findings demonstrate that CD3EDG humanized mice provide a powerful preclinical model for in vivo pharmacological efficacy studies of CD3-targeted antibodies. Values are expressed as mean ± SEM.
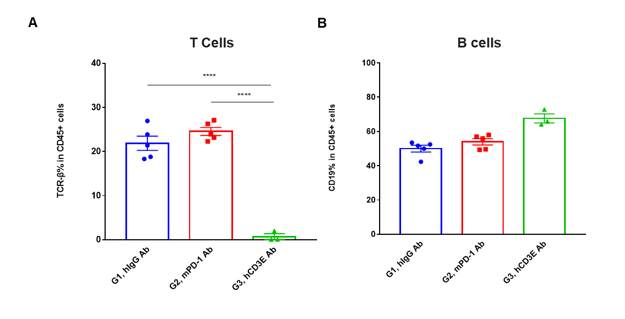
T cell and B cell activation in peripheral blood of CD3EDG humanized mice following in vivo antibody treatment. Lymphocytes were isolated from peripheral blood of homozygous CD3EDG humanized mice 48 hours after treatment. In the anti-human CD3ε antibody treatment group, the proportion of T cells was significantly reduced due to activation-induced cell death (AICD), while B cell proportions remained unaffected. (A) Percentage of TCR-β⁺ cells among total CD45⁺ cells after treatment. (B) Percentage of CD19⁺ cells among total CD45⁺ cells after treatment.
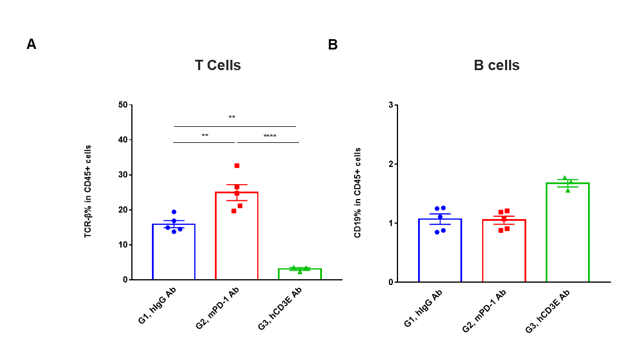
T cell and B cell activation in tumors of CD3EDG humanized mice following in vivo antibody treatment. Lymphocytes were isolated from tumors of homozygous CD3EDG humanized mice 48 hours after treatment. In the anti-human CD3ε antibody treatment group, the proportion of T cells was significantly reduced due to activation-induced cell death (AICD), while B cell proportions remained stable. (A) Percentage of TCR-β⁺ cells among total CD45⁺ cells after treatment. (B) Percentage of CD19⁺ cells among total CD45⁺ cells after treatment.
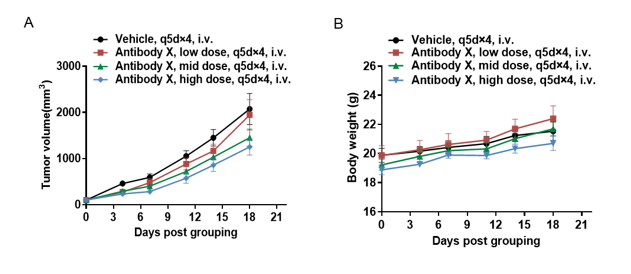
Antitumor activity of T cell bispecific antibody X in CD3EDG humanized mice. B-hBCMA MC38 tumor cells were subcutaneously implanted into female homozygous CD3EDG humanized mice (6-week-old, n=5). Mice were grouped when tumor volume reached approximately 100 mm³ and treated with antibody X provided by the client at the doses and schedules indicated. (A) Tumor volume changes during treatment. (B) Body weight changes during treatment. Antibody X at different doses effectively inhibited tumor growth in CD3EDG humanized mice, demonstrating that this model is a powerful tool for the in vivo efficacy evaluation of T cell bispecific antibodies. Values are expressed as mean ± SEM.
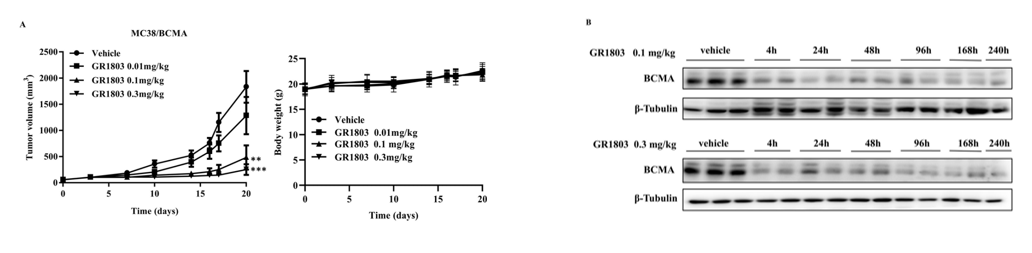
Antitumor efficacy of CD3 bispecific antibody GR1803 in CD3EDG humanized mice. Six- to eight-week-old female CD3EDG humanized mice (expressing human CD3E, CD3D, and CD3G) were purchased from Biocytogen Pharmaceuticals Co., Ltd. (Beijing, China) and subcutaneously implanted with MC38/BCMA tumor cells. Tumor-bearing mice were randomized into groups and intravenously (i.v.) injected with vehicle or GR1803 when average tumor volume reached approximately 50–100 mm³. Tumor volume was calculated as (length × width²)/2, and body weight was monitored as an indicator of health. (A) Tumor growth curves show that a single i.v. injection of GR1803 at 0.1 or 0.3 mg/kg significantly inhibited tumor growth compared with controls. (B) GR1803 treatment downregulated BCMA expression in tumor tissues beginning at 4 hours post-injection and persisting for at least 10 days. No significant body weight loss was observed, indicating that the treatment was well tolerated. Data are from the client's literature.
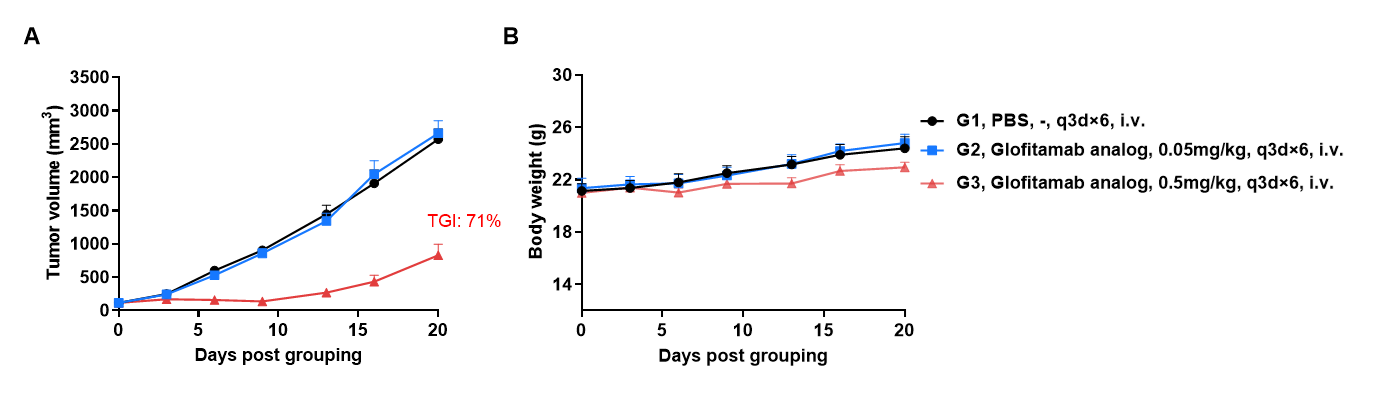
Antitumor activity of anti-human CD3 x CD20 bispecific antibody in CD3EDG humanized mice. B-hCD20 MC38 colon carcinoma cells were subcutaneously implanted into female homozygous CD3EDG humanized mice (7-week-old, n=6). Mice were grouped once tumor volume reached approximately 100 mm³ and treated intraperitoneally with an anti-human CD3/CD20 bispecific antibody (Glofitamab analog, commercially available) as indicated. (A) Anti-human CD3/CD20 bispecific antibody significantly inhibited tumor growth in CD3EDG humanized mice. (B) Body weight monitoring showed no significant changes during treatment, indicating good tolerability. Values are expressed as mean ± SEM.
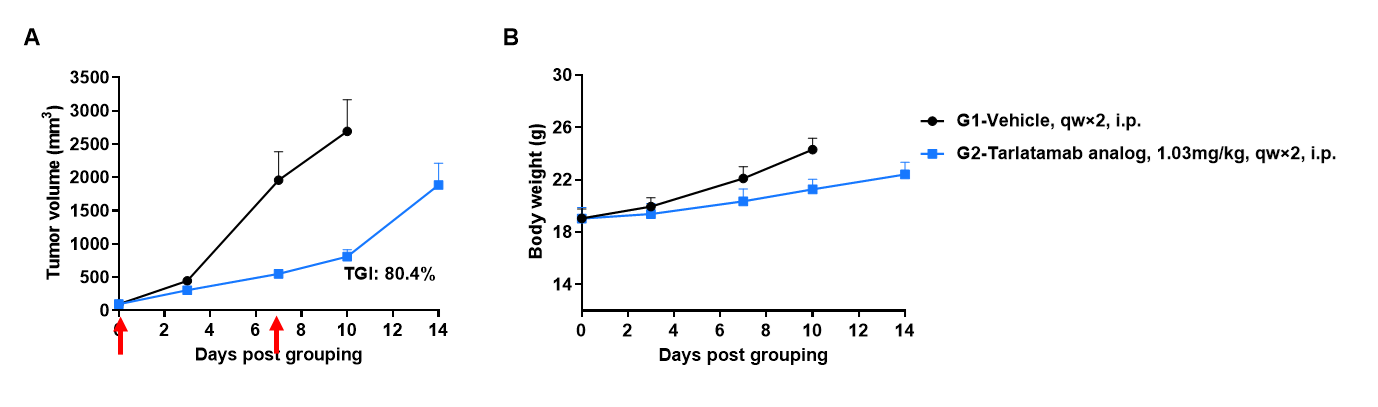
Antitumor activity of anti-human CD3 x DLL3 bispecific antibody in CD3EDG humanized mice. B-hDLL3 B16-F10 melanoma cells were subcutaneously implanted into female homozygous CD3EDG humanized mice (7–9 weeks old, n=6). Mice were grouped when tumor volume reached approximately 100 ± 20 mm³ and treated intraperitoneally with an anti-human CD3/DLL3 bispecific antibody (Tarlatamab analog, in-house) at the doses indicated. (A) Anti-human CD3/DLL3 bispecific antibody significantly inhibited B-hDLL3 B16-F10 tumor growth in CD3EDG humanized mice. (B) Body weight changes during treatment showed no significant differences, confirming tolerability. Values are expressed as mean ± SEM. Red arrows mark dosing time points.
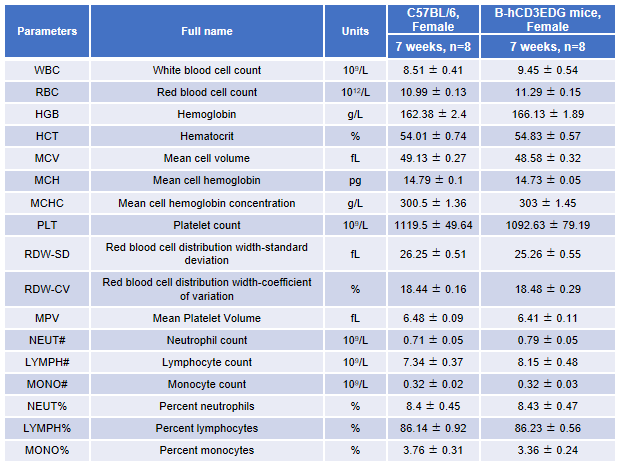
Complete blood count (CBC) analysis in CD3EDG humanized mice. Blood samples from female wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=8, 7-week-old) were collected and analyzed for complete blood count (CBC). No significant differences were observed in any hematological parameter between the two groups, indicating that replacement of mouse Cd3edg with human CD3EDG does not alter blood cell composition or morphology. Values are expressed as mean ± SEM.
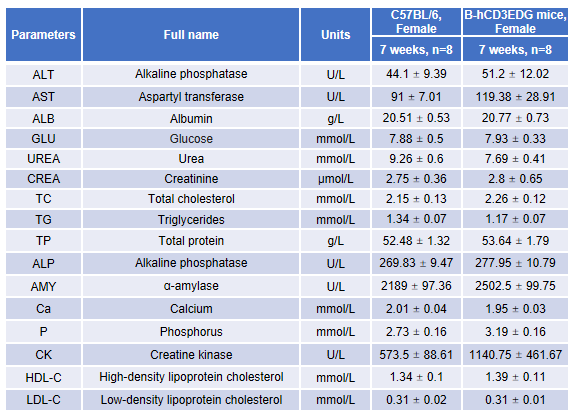
Blood chemistry analysis in CD3EDG humanized mice. Serum samples from female wild-type C57BL/6 mice and homozygous CD3EDG humanized mice (n=8, 7-week-old) were collected and analyzed for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. No significant differences were observed between the two groups, indicating that replacement of mouse Cd3edg with human CD3EDG does not alter ALT/AST levels or liver health. Values are expressed as mean ± SEM.
Q1: What are B-hCD3EDG mice?
A1: B-hCD3EDG mice are humanized models that express human CD3E, CD3D, and CD3G proteins. They provide a more predictive in vivo system for evaluating therapies targeting human CD3, which is critical for T cell activation and immunotherapy research.
Q2: Why use B-hCD3EDG mice instead of wild-type mice?
A2: Wild-type mice express murine CD3 chains that human antibodies may not recognize. B-hCD3EDG mice allow reliable testing of monoclonal antibodies, bispecifics, and T cell engagers directed at human CD3.
Q3: Do B-hCD3EDG mice have normal immune function?
A3: Yes. They maintain normal T, B, and NK cell development, with comparable immune cell distribution and T cell activation responses to wild-type mice.
Q4: What research areas benefit from B-hCD3EDG mice?
A4: These mice are valuable for immuno-oncology, antibody validation, T cell activation studies, and preclinical safety testing of CD3-directed therapies.