
C57BL/6-Glp1rtm1(GLP1R)Bcgen/Bcgen • 170164
在此页面上
GLP1R (Glucagon-like peptide-1 receptor) is a G protein–coupled receptor (GPCR) highly expressed in pancreatic β-cells and critical for glucose-dependent insulin secretion, appetite regulation, and energy balance. GLP1R is the therapeutic target of GLP-1 receptor agonists (GLP-1RAs), such as semaglutide and liraglutide, which are widely used for the treatment of type 2 diabetes and obesity.
In GLP1R humanized mice, the murine Glp1r gene is replaced with the human GLP1R gene. Homozygous mice express human GLP1R in physiologically relevant tissues without altering immune or metabolic homeostasis. This model provides a translationally relevant platform for preclinical efficacy testing, antibody validation, and drug development targeting GLP1R.
GLP1R humanized mice are an ideal preclinical model for evaluating GLP1R-targeted therapeutics in metabolic disease research, including type 2 diabetes and obesity.
Key Advantages
Validation
Applications
Gene targeting strategy for B-hGLP1R mice. The full coding sequence of human GLP1R gene was inserted into mouse Glp1r gene exon1 in B-hGLP1R mice. The human GLP1R protein expression will be driven by endogenous mouse Glp1r promoter, while mouse Glp1r gene transcription and translation will be disrupted.
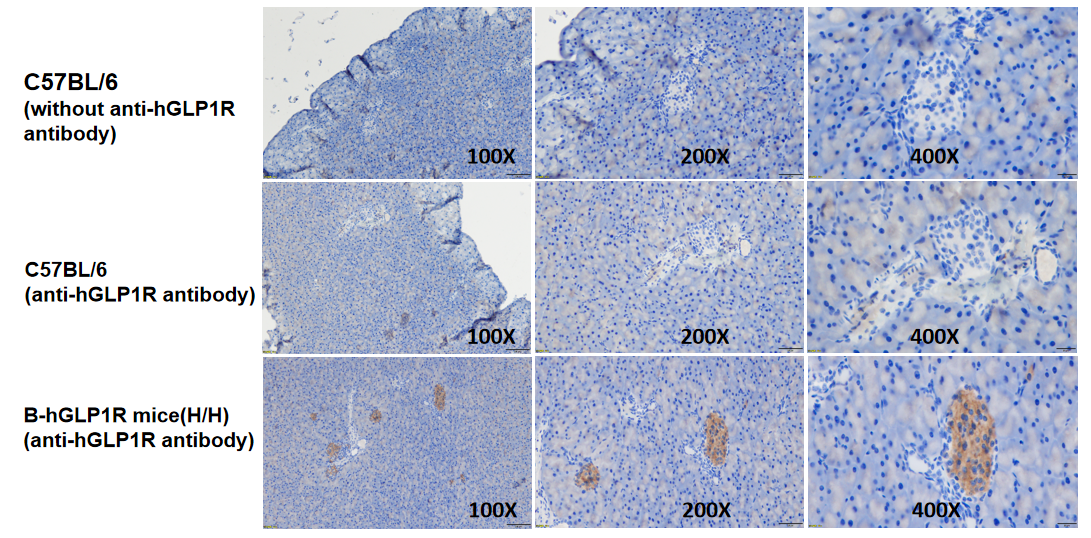
Human GLP1R expression detected by immunohistochemistry (IHC). Pancreatic tissues from wild-type (+/+) and GLP1R humanized mice (H/H) were stained with a human-specific GLP1R antibody (abcam, ab254352). Only the pancreas from GLP1R humanized mice showed positive membrane staining.
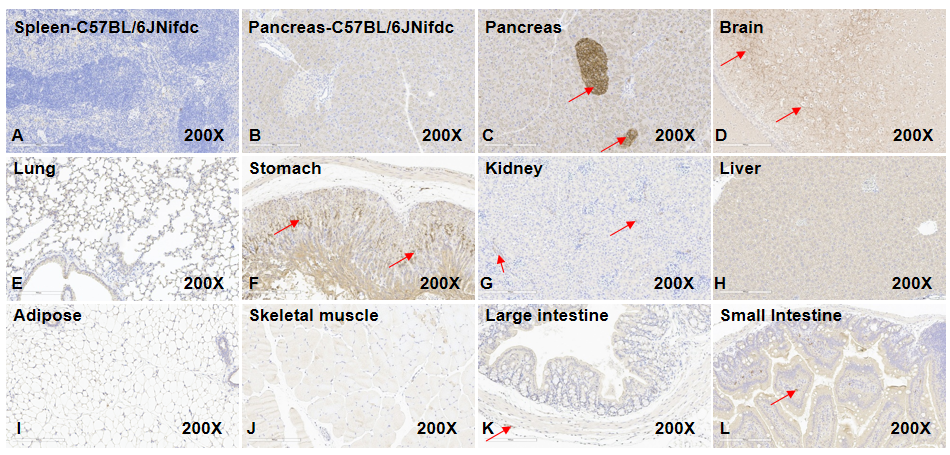
IHC analysis confirmed human GLP1R expression in multiple tissues of GLP1R humanized mice, including pancreatic islets, brain (striatum septum), gastric mucosa, kidney vasculature, myenteric plexus (small and large intestine), and intestinal interstitial cells. No human GLP1R signal was detected in lung, liver, adipose, or skeletal muscle. Wild-type tissues served as negative controls. (Original magnification ×200).
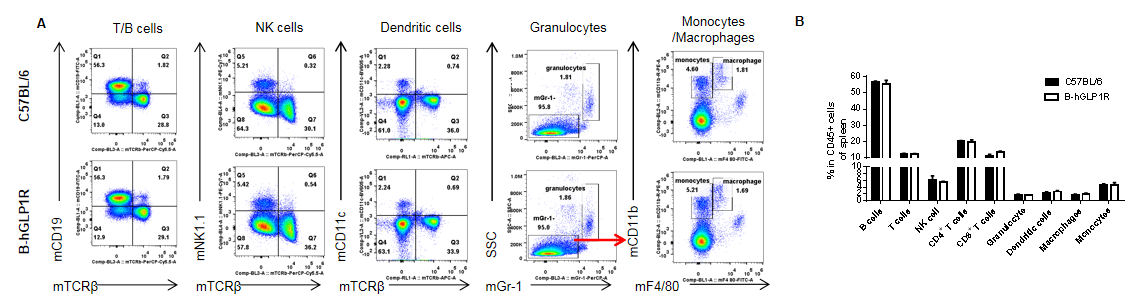
Analysis of spleen leukocyte subpopulations in GLP1R humanized mice. Splenocytes were isolated from female C57BL/6 and GLP1R humanized mice (n=3, 9 weeks old). Flow cytometry was performed to analyze leukocyte subpopulations. Single live cells were gated for the CD45⁺ population and further analyzed by FACS. Representative plots (A) and quantitative results (B) are shown. The percentages of T cells, B cells, NK cells, monocytes, dendritic cells, granulocytes, and macrophages in homozygous GLP1R humanized mice were comparable to those in wild-type C57BL/6 controls. Humanization of GLP1R does not alter the development, differentiation, or distribution of leukocyte subsets in the spleen. Values are expressed as mean ± SEM.
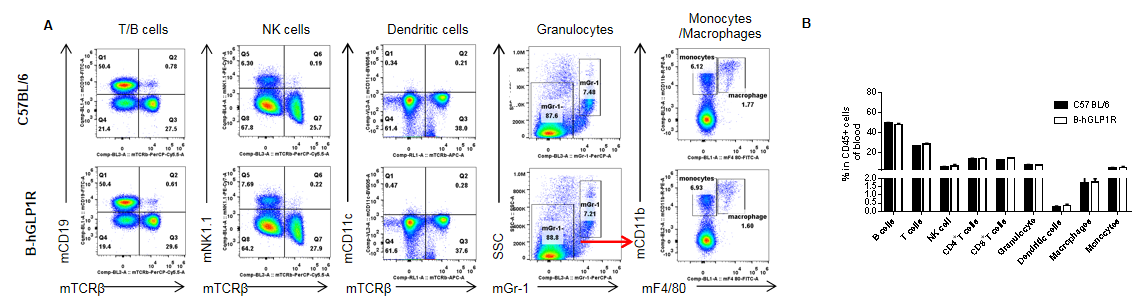
Analysis of blood leukocyte subpopulations in GLP1R humanized mice. Peripheral blood cells were isolated from female C57BL/6 and GLP1R humanized mice (B-hGLP1R, n=3, 9 weeks old). Flow cytometry was performed to evaluate leukocyte subpopulations. Single live cells were gated for the CD45⁺ population and analyzed by FACS. Representative plots (A) and quantification results (B) are shown. The percentages of T cells, B cells, NK cells, monocytes, dendritic cells, granulocytes, and macrophages in homozygous GLP1R humanized mice were comparable to those in C57BL/6 wild-type controls. Humanization of GLP1R does not affect the development, differentiation, or distribution of blood leukocyte subpopulations. Values are expressed as mean ± SEM.
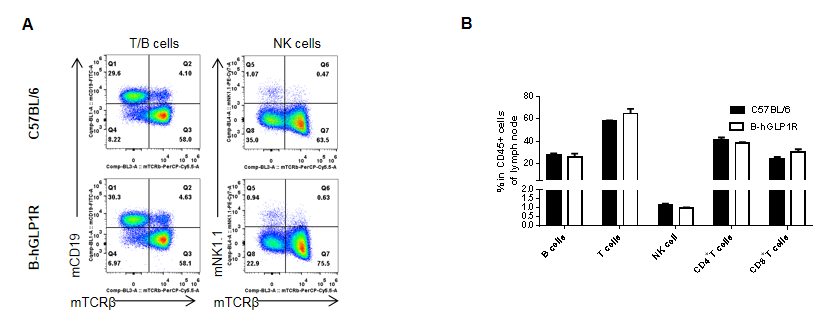
Analysis of lymph node leukocyte subpopulations in GLP1R humanized mice. Lymph nodes were isolated from female C57BL/6 and GLP1R humanized mice (B-hGLP1R, n=3, 9 weeks old). Flow cytometry was performed to evaluate leukocyte subpopulations. Single live cells were gated for the CD45⁺ population and analyzed by FACS. Representative plots (A) and quantitative results (B) are shown. The percentages of T cells, B cells, and NK cells in homozygous GLP1R humanized mice were comparable to those in wild-type C57BL/6 controls. Humanization of GLP1R does not affect the development, differentiation, or distribution of lymph node leukocyte subsets. Values are expressed as mean ± SEM.

Analysis of T cell subpopulations in GLP1R humanized mice. Lymphocytes were isolated from spleen, lymph nodes, and peripheral blood of female C57BL/6 and GLP1R humanized mice (B-hGLP1R, n=3, 9 weeks old). Flow cytometry was performed to assess T cell subpopulations. Cells were gated on the CD45⁺ population and analyzed for CD4⁺, CD8⁺, and regulatory T cell (Treg) subsets. The proportions of CD4⁺ T cells, CD8⁺ T cells, and Tregs in GLP1R humanized mice were comparable to those in wild-type controls across spleen, blood, and lymph nodes. Humanization of GLP1R does not alter the development, differentiation, or distribution of major T cell subsets in immune organs. Values are expressed as mean ± SEM.
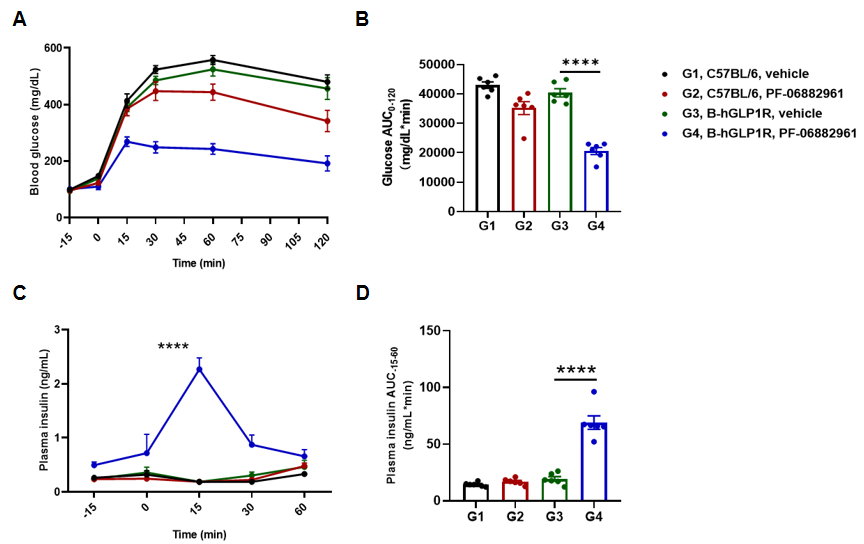
PF-06882961 improved glucose tolerance and promoted insulin secretion in GLP1R humanized mice. GLP1R humanized mice were treated with PF-06882961 (3 mg/kg, s.c.). An intraperitoneal glucose tolerance test (IPGTT, 2 g/kg D-glucose) was performed in both wild-type C57BL/6 and GLP1R humanized mice. Blood glucose (A), area under the curve (AUC) for IPGTT (B), plasma insulin (C), and AUC for plasma insulin (D). PF-06882961 significantly improved glucose tolerance and promoted insulin secretion in GLP1R humanized mice but had no effect in wild-type C57BL/6 mice. Values expressed as mean ± SEM. Significance determined by unpaired t test (*p<0.05, **p<0.01, ***p<0.0001). PF-06882961 exhibits human-specific activity by improving glucose tolerance and enhancing insulin secretion in GLP1R humanized mice, confirming their translational relevance for evaluating GLP-1 receptor agonists.
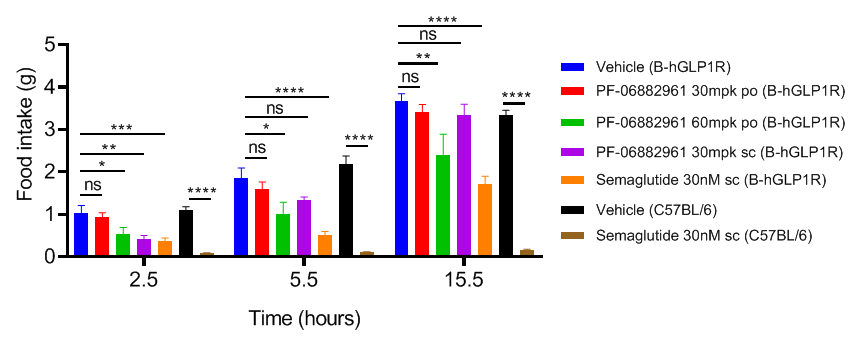
PF-06882961 and semaglutide effectively suppress food intake in GLP1R humanized mice. GLP1R humanized mice (12 weeks old) were acclimated to handling and oral gavage with vehicle for 3 days prior to the study. Mice were grouped according to body weight and baseline daily food intake. Treatments were administered at the indicated dose and route. Food intake was recorded at 2.5, 5.5, and 15.5 hours post-treatment. Values are expressed as mean ± SEM. Significance determined by one-way ANOVA (*p<0.05, **p<0.01, ***p<0.0001). PF-06882961 and Semaglutide effectively suppress food intake in GLP1R humanized mice, validating this model for preclinical evaluation of GLP-1 receptor–targeted anti-obesity therapeutics.
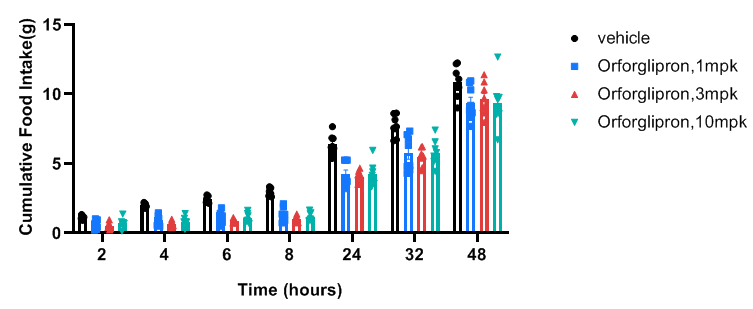
Orforglipron suppresses food intake in GLP1R humanized mice. GLP1R humanized mice (9 weeks old) were acclimated for once-daily (QD) oral dosing with vehicle for 4 days prior to the study. Mice were grouped according to body weight and baseline food intake. After overnight fasting, mice received vehicle or Orforglipron by oral gavage in the morning (before 10 AM). Food was returned 15 minutes post-dosing. Food consumption was recorded at 2, 4, 6, 8, 24, 32, and 48 hours post-treatment. Orforglipron significantly reduced food intake in GLP1R humanized mice compared with vehicle controls, with effects sustained up to 48 hours, confirming the model’s value for preclinical evaluation of GLP-1 receptor agonists for obesity and metabolic disorders. Values are expressed as mean ± SEM.

Semaglutide effectively reduces body weight and suppresses food intake in diet-induced obese GLP1R humanized mice. GLP1R humanized mice (study ID: 23P042001) were fed a high-fat diet (HFD) for 12 weeks to establish an obesity model. (A) Body weight changes after HFD induction. (B–D) Body weight reduction following Semaglutide treatment. (E–F) Effects of Semaglutide on food intake. Semaglutide significantly reduced body weight and food intake in HFD-induced obese GLP1R humanized mice, confirming their translational relevance for preclinical obesity drug development. 8–10 mice per group. Values expressed as mean ± SEM. Significance determined by one-way ANOVA (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
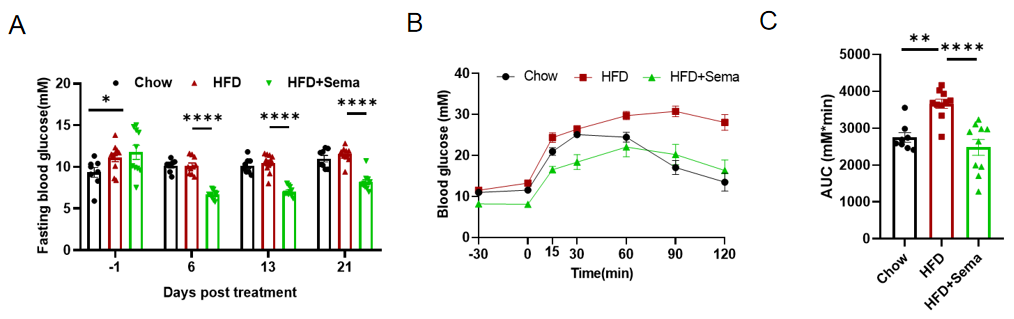
Glucose Regulation by Semaglutide in HFD-Induced GLP1R Humanized Mice. GLP1R humanized mice were fed a high-fat diet (HFD) to induce obesity and metabolic dysfunction. Following 12 weeks of HFD feeding, mice were treated with Semaglutide. (A) Blood glucose changes after Semaglutide treatment. (B) Intraperitoneal glucose tolerance test (IPGTT, 2 g/kg D-glucose). (C) Area under the curve (AUC) for IPGTT. Semaglutide significantly lowered blood glucose levels and improved glucose tolerance in obese GLP1R humanized mice compared with vehicle controls. AUC analysis further confirmed the enhanced glycemic control. 8–10 mice per group. Values expressed as mean ± SEM. Significance determined by one-way ANOVA (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001). Semaglutide improves glucose regulation and insulin sensitivity in diet-induced obese GLP1R humanized mice, supporting their use in preclinical studies of diabetes and metabolic diseases.
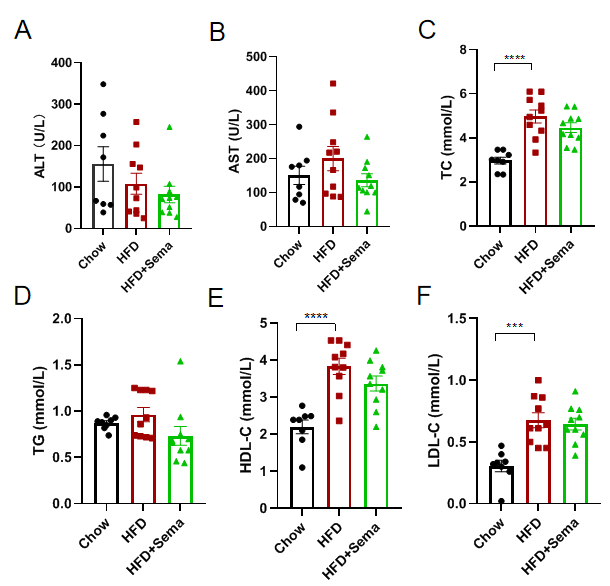
Semaglutide improved blood biochemistry in obese GLP1R humanized mice. GLP1R humanized mice were fed a high-fat diet (HFD) for 12 weeks to induce obesity and metabolic alterations. Following Semaglutide treatment, blood biochemistry was analyzed. Multiple serum biochemical parameters were assessed (A–F). Semaglutide significantly improved blood biochemical markers in obese GLP1R humanized mice compared with vehicle-treated controls, reflecting enhanced metabolic function. 8–10 mice per group. Values expressed as mean ± SEM. Significance determined by one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). Semaglutide improves metabolic homeostasis in HFD-induced obese GLP1R humanized mice, demonstrating the model’s translational relevance for evaluating GLP-1 receptor–targeted therapies in obesity and type 2 diabetes.
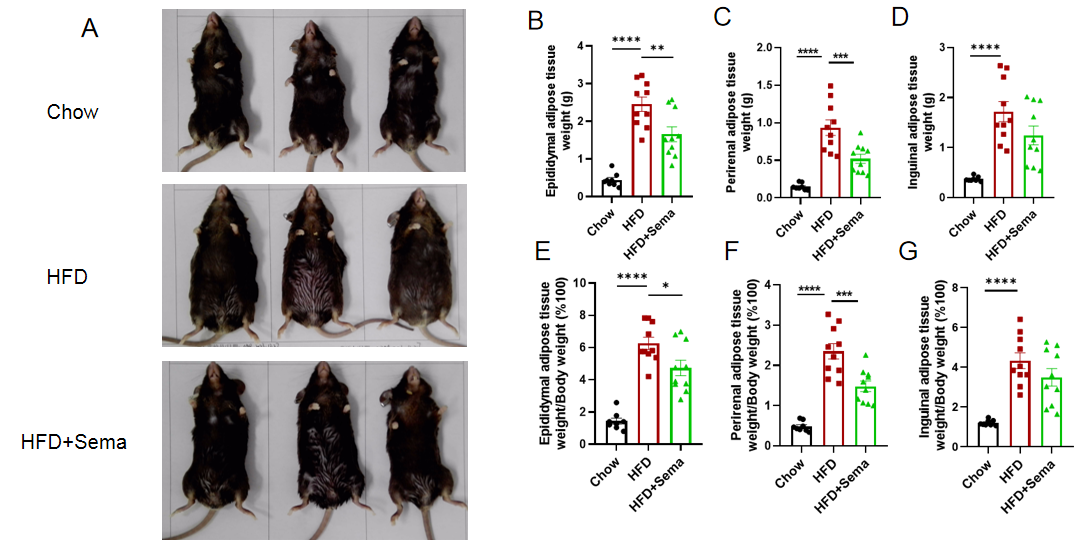
Semaglutide reduces fat accumulation and adiposity in obese GLP1R humanized mice. GLP1R humanized mice (B-hGLP1R) were fed a high-fat diet (HFD) for 12 weeks to establish an obesity model. After Semaglutide treatment, adipose tissue weight and distribution were analyzed. (A) Representative images of experimental groups at study termination. (B–D) Adipose tissue weights after treatment. (E–G) Ratios of adipose tissue weight to total body weight. Semaglutide significantly reduced adipose tissue mass and adiposity ratios in obese GLP1R humanized mice compared with vehicle-treated controls, validating this model for preclinical evaluation of GLP-1 receptor agonists in obesity management. 8–10 mice per group. Values expressed as mean ± SEM. Significance determined by one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
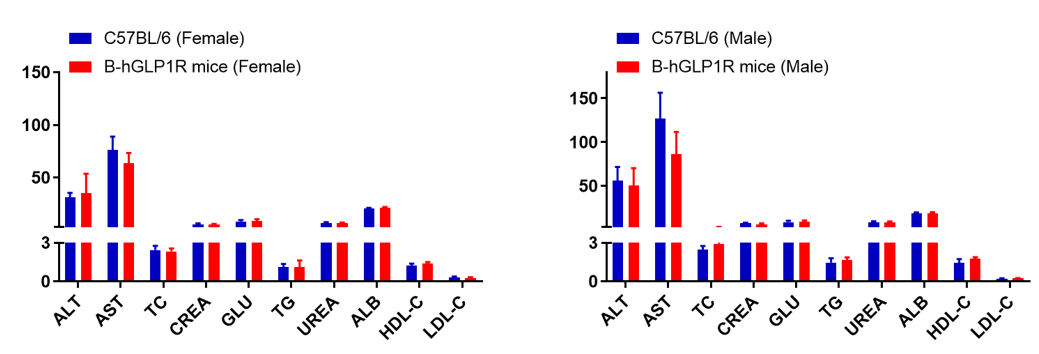
Serum from wild-type and GLP1R humanized mice (n=6, 6–8 weeks old) was analyzed. No significant differences were observed in biochemical indicators between humanized and wild-type mice. Values expressed as mean ± SEM.
Q1: What are GLP1R humanized mice?
A1: GLP1R humanized mice are genetically engineered models in which the murine Glp1r gene is replaced with the human GLP1R gene. These mice express human GLP-1 receptor (GLP1R) in physiologically relevant tissues such as pancreatic islets and gut endocrine cells, while maintaining normal immune and metabolic function.
Q2: Why are GLP1R humanized mice important for diabetes and obesity research?
A2: GLP1R is the therapeutic target of GLP-1 receptor agonists (GLP-1RAs), including Semaglutide and Liraglutide. GLP1R humanized mice provide a reliable preclinical model to evaluate drug efficacy, safety, and pharmacokinetics in vivo, supporting the development of treatments for type 2 diabetes, obesity, and metabolic syndrome.
Q3: Can GLP1R humanized mice be used for antibody validation?
A3: Yes. Because these mice express human GLP1R, they enable in vivo antibody validation and testing of GLP-1 receptor–targeted biologics. Researchers can assess antibody binding, efficacy, and safety in a translationally relevant system.
Q4: How do GLP1R humanized mice differ from wild-type mice?
A4: Unlike wild-type C57BL/6 mice, GLP1R humanized mice express human GLP1R exclusively. Importantly, their immune cell distribution, blood biochemistry, and baseline metabolic parameters remain normal, ensuring that experimental results are attributable to human GLP1R function rather than systemic alterations.
Q5: What disease models can be established using GLP1R humanized mice?
A5: GLP1R humanized mice have been validated in: